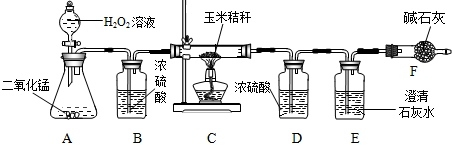
��Ŀ����
ij�о�С����̽�������Ȼ������ʵĴ����Ʒ��̼���Ƶ�������������ȡ25g������Ʒ����������ˮ�У������õ��Ļ����Һ����μ���������������Ϊ22.2%��CaCl2��Һ����¼����ͼ��ʾ�����߹�ϵ��
����֪��CaCl2+Na2CO3=2NaCl+CaCO3����
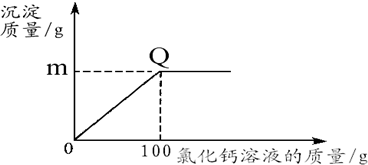
��1��������Q������
��2��ͼ��m��ֵΪ���ٿˣ���
��3����Ʒ���Ȼ��Ƶ���������Ϊ���٣���������д��������̣�
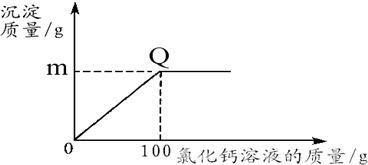
����֪��CaCl2+Na2CO3=2NaCl+CaCO3����
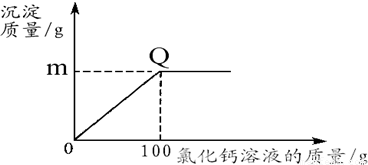
��1��������Q������
��Ʒ�е�̼�������Ȼ�����Һǡ����ȫ��Ӧ
��Ʒ�е�̼�������Ȼ�����Һǡ����ȫ��Ӧ
����2��ͼ��m��ֵΪ���ٿˣ���
��3����Ʒ���Ȼ��Ƶ���������Ϊ���٣���������д��������̣�

��������1�����ݷ�Ӧԭ������Q�㺬��
��2�����ݻ�ѧ����ʽ����֪�Ȼ��Ƶ�������������ɵ�̼��Ƶ�����
��3�����ݻ�ѧ����ʽ����֪�Ȼ��Ƶ������������̼���Ƶ�����������������ȥ̼����������Ϊ�Ȼ����������Ӷ�����Ȼ��Ƶ�����������
��2�����ݻ�ѧ����ʽ����֪�Ȼ��Ƶ�������������ɵ�̼��Ƶ�����
��3�����ݻ�ѧ����ʽ����֪�Ȼ��Ƶ������������̼���Ƶ�����������������ȥ̼����������Ϊ�Ȼ����������Ӷ�����Ȼ��Ƶ�����������
����⣺��1��Q���ʾ��Ʒ�е�̼�������Ȼ�����Һǡ����ȫ��Ӧ
��2����̼�������Ȼ���ǡ����ȫ��Ӧʱ����̼��Ƶ�����Ϊm����Ʒ��̼��������Ϊx
Na2CO3 +CaCl2 �TCaCO3��+2NaCl
106 111 100
x 100g��22.2% m
=
m=10g
��3��
=
x=10.6g
��Ʒ���Ȼ��Ƶ���������Ϊ
��100%=57.6%
�𣺣�2��m��ֵΪ10g
��3����Ʒ���Ȼ��Ƶ���������Ϊ57.6%
��2����̼�������Ȼ���ǡ����ȫ��Ӧʱ����̼��Ƶ�����Ϊm����Ʒ��̼��������Ϊx
Na2CO3 +CaCl2 �TCaCO3��+2NaCl
106 111 100
x 100g��22.2% m
| 111 |
| 100 |
| 100g��22.2% |
| m |
m=10g
��3��
| 106 |
| 111 |
| x |
| 100g��22.2% |
��Ʒ���Ȼ��Ƶ���������Ϊ
| 25g-10.6g |
| 25g |
�𣺣�2��m��ֵΪ10g
��3����Ʒ���Ȼ��Ƶ���������Ϊ57.6%
������������Ҫ����ѧ������ȫ��Ӧ����ʶ���Լ����û�ѧ����ʽ������������ʽ���м����������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ