��Ŀ����
����Ŀ��ʵ���������ṩ��ҩƷ�и�����ء��������̡�ʯ��ʯ������������Һ��ϡ���ᡢϡ���ᡢˮ�Լ���ͼ����������ش��������⡣

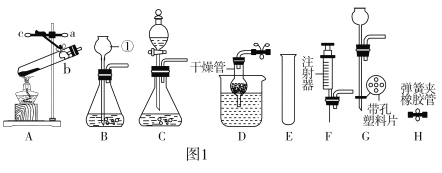
��1�����Bװ�������Եķ����ǣ����ü��Ӽ�ס�뵼�������ӵĽ�Ƥ�ܣ�����a�м���ˮ���γ�һ��ˮ�������ã����۲쵽___________��˵�����������á�
��2��ѡ������װ�ú�ҩƷ��ȡһƿ������̼����ѡ���װ����_______(����ĸ���)��
��3��������ȡ������̼װ�ã�������������ȡ��������Ӧ�Ļ�ѧ����ʽΪ__________��
��4���ø��������ȡ������������ˮ���ռ���Ӧѡ�õķ���װ����___������ĸ��ţ�����Ӧ�Ļ�ѧ����ʽΪ___________________��
���𰸡�һ��ʱ���ˮ���߶Ȳ��� AE��BE 2H2O2![]() 2H2O +O2�� C 2KMnO4
2H2O +O2�� C 2KMnO4![]() K2MnO4 + MnO2 +O2��
K2MnO4 + MnO2 +O2��
��������
��1�����Bװ�������Եķ����ǣ����ü��Ӽ�ס�뵼�������ӵĽ�Ƥ�ܣ�����a�м���ˮ���γ�һ��ˮ�������ã����۲쵽һ��ʱ���ˮ���߶Ȳ��䣬˵�����������ã�
��2��ʵ������ȡ������̼�ķ�Ӧ���ǹ����Һ�壬��Ӧ�����dz��£�������̼�ܶȱȿ���������ˮ������ѡ���װ����AE��BE��
��3�����������ڶ������̵Ĵ������·ֽ�����ˮ����������ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����
��4��ʵ�����ø��������ȡ�����ķ�Ӧ���ǹ��壬��Ӧ�����Ǽ��ȣ�����Ӧѡ�õķ���װ����C����������ڼ��ȵ���������������ء��������̺���������ѧ����ʽΪ��2KMnO4![]() K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ղ�����������ʵ����̽����
��֪ʶ�عˣ�
��1��д����ʵ���У��ƾ���C2H6O��ȼ������ˮ�Ͷ�����̼�Ļ�ѧ����ʽ��_____��
��2������δȼ�յ�ԭ����_____��
��������⣩����ֽ����������ʵ�飬�ʺϵľƾ���Һ��������������Χ�Ƕ���?
��ʵ�鲽�裩��.����ͼ�������Ʋ�ͬŨ�ȵľƾ���Һ����֪:��ˮ�ƾ����ܶ�Ϊ0.8g/mL��ˮ���ܶ�Ϊ1.0g/mL��
ʵ���� | ��ˮ�ƾ����/mL | ˮ���/mL |
�� | 10 | 5 |
�� | 10 | 10 |
�� | 10 | 15 |
�� | 10 | 20 |
�� | 10 | 30 |
��.�ò�ͬŨ�ȵľƾ���Һ������ֽ��
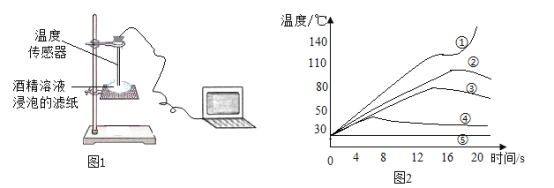
��.����ͼ1��ʾװ�òⶨ�ƾ�ȼ��ʱ��ֽ���¶ȣ��������ݻ��Ƴ�ͼ2��
�Իش�:
��3�����ߢ��ڽӽ�20sʱ���¶�ͻȻ��������Ҫԭ����_____
��4��ʵ��۴�Լ��_____��ʱ�ƾ�ȼ��������Ϩ��
��ʵ����ۣ�
��5������ͼ2��Ϣ������ֽ�����ղ�����������ʵ�飬Ϊȷ��ʵ��ɹ������þƾ���Һ��������������������������Χ��_____�����ռ�����������С�����1λ��
����˼��ߣ�
��6������ͼ2�����ߢܺ͢ݣ��������ȼ������������ʶ��_____��
��7����ʵ��ڵľƾ���Һʵ��ʱ������ֽ�������һ�ţ������ֽ���ճɻҽ�������Ҫ��Ũ�Ƚϴ�ľƾ���Һ�ɹ���ɱ�ʵ��Ӧ��_____��
����Ŀ��С��ͬѧ�������������Ľ������µ�ʵ�顣

ʵ��һ����ͼA����ȼ����һ��Сľ�����������У�Լ1s��ȡ������������˵����_____��
ʵ�������ͼB��������б����һ��ϸ�����ܣ��ڲ������Ͽڵ�ȼ���������棬��˵�����Ĵ������ʵ�������_____��дһ�㣩��
����̽�������Ĵ�������������ʲô��
�����ϣ�����IJ���ȫȼ�ջ����һ����̼��һ����̼��ʹ�����պڵ���ͭ˿��졣
��ʵ��̽����
���� | ���� | ���� | ���� |
����A������������CO2�� | ���������嵼����ͨ�����ʯ��ˮ�� | _____ | ����A������ |
����B������������_____�� | �ñ����պڵ���ͭѸ�ٲ���ͼBϸ�������� | �����պڵ���ͭ˿��� | _____ |
����C������������ʯ������ | ��1��ȡһ���ϳ���ϸ�����ܣ���ʪ��ë����ס�в������������¶˲������ģ��϶����û���ȼ�� ��2��ȡ��ϸ�����ܣ���ʪ��ë�� | ��1��_____�� ��2��_____�� | ����C���� |