��Ŀ����
ʵ��С���о����ᡢ���������������ʵĻ�ѧ���ʣ���������ͼ��ʾ8��ʵ�顣

��֪��Na2CO3��CaCl2=CaCO3����2NaCl
�� ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թ��з�����Ӧ�Ļ�ѧ����ʽΪ___________________��
�� ʵ���ij�Թ���Ϊ��ɫ��Һ�������м���������________����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е�������___________��
�� ʵ���![]() ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬһ��ʱ��������ݳ��֡��ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����Ϊ______________��
ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬһ��ʱ��������ݳ��֡��ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����Ϊ______________��
�� ʵ���ij�Թ���ֻ�õ���ɫ��Һ�������м���������Na2CO3��Һ�������������ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����ʽΪ__________��ԭ��ɫ��Һ�е�������________��
(1)Fe2O3+6HCl=2FeCl3+3H2O
(2)ϡ��![]() ���ɫ��̪��Һ
���ɫ��̪��Һ
(3)Ca(OH)2+Na2CO3=CaCO3��+2NaOH
(4)Na2CO3+2HCl=2NaCl+H2O+CO2����Na2CO3��Na2CO3��NaCl

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
 ij����С��������þ�Ͻ�����о����ⶨ����þ������������
ij����С��������þ�Ͻ�����о����ⶨ����þ���������������������ϡ�
��1��������þ������������������ˮ�����ܼ����Ⱥ��ֽܷ�����ˮ����Ӧ�Ľ��������
��2��þ��������þ������������������Һ��Ӧ����������������������������������Һ�������·�Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2�� Al��OH��3+NaOH=NaAlO2+2H2O
��������ơ���������ʵ�����ṩ�����ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ�
| ||
����������þ�Ͻ�
| ||
����������þ�Ͻ�
| ||
| ||
���������ۡ�
1�����������Ƿ�����У����в����е���˵�����ɣ�
2���������С���Ա����Ը����ú��ַ�����
��ʵ����ơ�ʵ��С����ݷ��������������ͼ��ʾ��ͼ�е�����̨��ʡ�ԣ�������ʵ��װ�ã�
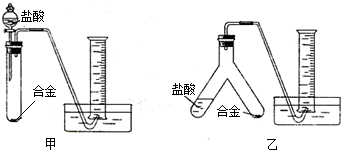
����Ϊѡ��
���������ۡ�
��1��С���Աʵ���ͨ�����������ձ���Ϊ��������ʵ�鷽�������ڲ��������������ײ��������������⣮���ǰ���������ʽ��������˷��������������������·�������ƣ��ڡ��Ϸ���д��Ӧ���Լ��Ͳ�������
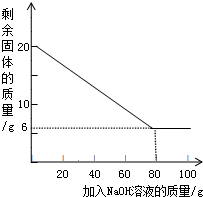
2��С���Ա�����µIJⶨ������ȡ20g��þ�Ͻ��гɽ�����ĩ��100g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�
| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ʣ����������/g | 1 6.5 | n | 9.5 | �� |
��2�������������ݿ�֪���ϱ���n��ֵΪ
��3���ý�����ĩ��Al����������Ϊ
��4����ʽ���㣺��������������Һ��������������Ϊ���٣�������̣�
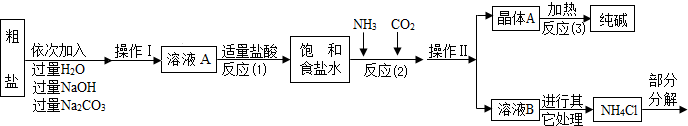
ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
[��������]
������1���ó����á������Ƽ�������Ļ�����ƷΪ�����ѧʽΪ �����Ȼ�泥�
���������Ƽ�����ҹ�����������ѧ�Һ�°�����һ�����������������Ȼ�淋������Ƽ�գ���
������2������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
������3������������������ͼ��ʾ��
�����������漰�IJ��ַ�Ӧ�Ļ�ѧ����ʽΪ��MgCl2+2NaOH=Mg��OH��2��+2NaCl��
Na2CO3+CaCl2=CaCO3��+2NaCl��NH4Cl�TNH3��+HCl��
����ԭ������������NH3���Ͷ�����̼ͨ�뱥��ʳ��ˮ�еõ�С�մ���Ȼ�淋Ļ����䷴Ӧ�Ļ�ѧ����ʽΪ��NaCl��������Һ��+NH3+CO2+H2O=NaHCO3�����壩��+NH4Cl�������NaHCO3��ʹ�������ȼ��ɷֽ��Ƶô�����ֳ�����������˷�Ӧ�Ļ�ѧ����ʽΪ ��
���������ۡ�
��1����ҺA�е�����Ϊ �������������Ϊ ��
��2���������������п�ѭ��ʹ�õ��� ������ţ���
A��CO2B��NH3C��HCl D��NaOH E��Na2CO3
��3������������Ϣ���������ʵ������̼���ƺ�̼�����ƣ�
�������ⶨ��
ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飮

���飺ȡ10.0g������Ʒ���ٶ�����������ʵ������в������仯���������м�����������ᣬֱ����Ʒ��������ð������ּ��������������ʲ������ȴ�����º���������ù�������Ϊ10.9g����Ʒ��̼���Ƶ���������Ϊ ���������һλС������
���飺ȡ10.0g������Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C������3.5g�������Լ�����������
ʵ�������������ⶨ����������ƫС����ԭ���� ��
[��������]
������1���ó����á������Ƽ�������Ļ�����ƷΪ�����ѧʽΪ
���������Ƽ�����ҹ�����������ѧ�Һ�°�����һ�����������������Ȼ�淋������Ƽ�գ���
������2������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
������3������������������ͼ��ʾ��

�����������漰�IJ��ַ�Ӧ�Ļ�ѧ����ʽΪ��MgCl2+2NaOH=Mg��OH��2��+2NaCl��
Na2CO3+CaCl2=CaCO3��+2NaCl��NH4Cl�TNH3��+HCl��
����ԭ������������NH3���Ͷ�����̼ͨ�뱥��ʳ��ˮ�еõ�С�մ���Ȼ�淋Ļ����䷴Ӧ�Ļ�ѧ����ʽΪ��NaCl��������Һ��+NH3+CO2+H2O=NaHCO3�����壩��+NH4Cl�������NaHCO3��ʹ�������ȼ��ɷֽ��Ƶô�����ֳ�����������˷�Ӧ�Ļ�ѧ����ʽΪ
���������ۡ�
��1����ҺA�е�����Ϊ
��2���������������п�ѭ��ʹ�õ���
A��CO2B��NH3C��HCl D��NaOH E��Na2CO3
��3������������Ϣ���������ʵ������̼���ƺ�̼�����ƣ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飮

���飺ȡ10.0g������Ʒ���ٶ�����������ʵ������в������仯���������м�����������ᣬֱ����Ʒ��������ð������ּ��������������ʲ������ȴ�����º���������ù�������Ϊ10.9g����Ʒ��̼���Ƶ���������Ϊ
���飺ȡ10.0g������Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C������3.5g�������Լ�����������
ʵ�������������ⶨ����������ƫС����ԭ����