��Ŀ����
���������ʵ��װ��ͼ�ش����⣺

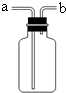
��1��д�����a��b���������ƣ�a_________��b_________��
��2���ø��������ȡ������Ӧѡ�÷���װ��_______���ռ�װ��_______�����ţ���������Ӧ�Ļ�ѧ����ʽΪ________________________������������ķ�����________________________��
��3��ʵ������ȡ������һ�㲽���У���װҩƷ�ڼ�������Ԣ۹̶�װ�âܼ��Ȣ�Ϩ��ƾ��Ƣްѵ��ܴ�ˮ�����ó����ռ����塣��ȷ��ʵ����������ǣ�_______��
A���٢ڢۢܢߢݢ� B. �ڢ٢ۢܢߢޢ� C. �٢ڢۢܢߢޢ� D. �ڢ٢ۢܢߢݢ�
��4�����л�ѧ�У���Aװ�������巢��װ�ÿ�����ȡ_______(��һ����������)���䷢����Ӧ�Ļ�ѧ����ʽΪ______________________��
��2���ø��������ȡ������Ӧѡ�÷���װ��_______���ռ�װ��_______�����ţ���������Ӧ�Ļ�ѧ����ʽΪ________________________������������ķ�����________________________��
��3��ʵ������ȡ������һ�㲽���У���װҩƷ�ڼ�������Ԣ۹̶�װ�âܼ��Ȣ�Ϩ��ƾ��Ƣްѵ��ܴ�ˮ�����ó����ռ����塣��ȷ��ʵ����������ǣ�_______��
A���٢ڢۢܢߢݢ� B. �ڢ٢ۢܢߢޢ� C. �٢ڢۢܢߢޢ� D. �ڢ٢ۢܢߢݢ�
��4�����л�ѧ�У���Aװ�������巢��װ�ÿ�����ȡ_______(��һ����������)���䷢����Ӧ�Ļ�ѧ����ʽΪ______________________��
(1)����©��������ƿ
(2)C��E��2KMnO4 K2MnO4+MnO2+O2�����������ǵ�ľ�����뼯��ƿ��
K2MnO4+MnO2+O2�����������ǵ�ľ�����뼯��ƿ��
(3)B
(4)������2H2O2 2H2O��+O2��(��������Zn+H2SO4==ZnSO4+H2���������̼��CaCO3+2HCl==CaCl2+H2O+CO2��)
2H2O��+O2��(��������Zn+H2SO4==ZnSO4+H2���������̼��CaCO3+2HCl==CaCl2+H2O+CO2��)
(2)C��E��2KMnO4
 K2MnO4+MnO2+O2�����������ǵ�ľ�����뼯��ƿ��
K2MnO4+MnO2+O2�����������ǵ�ľ�����뼯��ƿ��(3)B
(4)������2H2O2
 2H2O��+O2��(��������Zn+H2SO4==ZnSO4+H2���������̼��CaCO3+2HCl==CaCl2+H2O+CO2��)
2H2O��+O2��(��������Zn+H2SO4==ZnSO4+H2���������̼��CaCO3+2HCl==CaCl2+H2O+CO2��)
��ϰ��ϵ�д�
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ