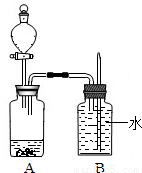
��Ŀ����
��2008?���ݣ�ijС��������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ��������ǻ�������ͭ��Һ������Ϊ��֤ʵ���룬��������ʵ�飺��1��ȡ8.5g���۷���һ����������ͭ��Һ�У���Ӧ��ɺ��˳����壬ϴ�ӡ�������������������Ϊ9.2g��
��2����9.2g������������ϡ�����ַ�Ӧ��Ӧ����������������Ϊ0.1g����
����ϡ���ᷴӦ������������
��������ͭ��Ӧ������������
��������ͭ��Ӧ�����������������ᷴӦ����������֮��______8.5g������ڡ�����С�ڡ����ڡ������Դν��������Ľ���______��
���𰸡���������1�������������ᷴӦ�Ļ�ѧ����ʽ�����������������������������
��2��9.2g�����������ᷢ����Ӧ��˵��9.2g���岻ȫ��ͭ������������9.2g-�����л��е���������=ͭ��������������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ�������������������
��3��������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ�����˵������ͭ��Һ�л����ᣬ������������ͭ��Һ�е��ᷢ����Ӧ�����Ի����������ͭ��Ӧ�����������������ᷴӦ����������֮��С��8.5g��
����⣺��1���������ᷴӦ����������Ϊx
Fe+2HCl=FeCl2+H2��
56 2
x 0.1g
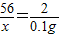
x=2.8g ����ϡ���ᷴӦ������������2.8g��
��2��9.2g������ͭ������Ϊ��9.2g-2.8g=6.4g
��������ͭ��Ӧ����������Ϊy
Fe+CuSO4=FeSO4+Cu
56 64
y 6.4g
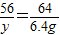
y=5.6g �𣺺�����ͭ��Ӧ����������Ϊ5.6g��
��3��2.8g+5.6g=8.4g��8.5g
�ʴ�Ϊ��С�� ��Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
������������ͭ������Ӧ��ʵ��ʱ�����ɵ�ͭ���������ı��棬�������������ȫ��Ӧ�����Խ����ɵ�ͭ���������л����������ɣ�����ʵ��δ��Ӧ�����������ᷢ����Ӧ��
��2��9.2g�����������ᷢ����Ӧ��˵��9.2g���岻ȫ��ͭ������������9.2g-�����л��е���������=ͭ��������������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ�������������������
��3��������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ�����˵������ͭ��Һ�л����ᣬ������������ͭ��Һ�е��ᷢ����Ӧ�����Ի����������ͭ��Ӧ�����������������ᷴӦ����������֮��С��8.5g��
����⣺��1���������ᷴӦ����������Ϊx
Fe+2HCl=FeCl2+H2��
56 2
x 0.1g
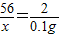
x=2.8g ����ϡ���ᷴӦ������������2.8g��
��2��9.2g������ͭ������Ϊ��9.2g-2.8g=6.4g
��������ͭ��Ӧ����������Ϊy
Fe+CuSO4=FeSO4+Cu
56 64
y 6.4g
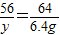
y=5.6g �𣺺�����ͭ��Ӧ����������Ϊ5.6g��
��3��2.8g+5.6g=8.4g��8.5g
�ʴ�Ϊ��С�� ��Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
������������ͭ������Ӧ��ʵ��ʱ�����ɵ�ͭ���������ı��棬�������������ȫ��Ӧ�����Խ����ɵ�ͭ���������л����������ɣ�����ʵ��δ��Ӧ�����������ᷢ����Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��2008?���ݣ�ijѧУ��ȤС�����о���������ľ����ȼ��������������Ĺ�ϵ���Ŀ����У���ȡ������ʵ�鲽�裺
���÷ֽ�������⣨H2O2����ȡһ������������Ȼ�����ij�ַ����õ�5ֻ����ƿ�����250mL��������������������α��Ϊ1��2��3��4��5��������ǵ�ľ�����β���1��5��ƿ�У��ѹ۲쵽����ͼ������ݣ������±���
�Իش��������⣺
��1��ʵ�����в��÷ֽ�������⣨H2O2���ķ�����ȡ�������䷴Ӧ�Ļ�ѧ����ʽ��______ 2H2O+O2��
���÷ֽ�������⣨H2O2����ȡһ������������Ȼ�����ij�ַ����õ�5ֻ����ƿ�����250mL��������������������α��Ϊ1��2��3��4��5��������ǵ�ľ�����β���1��5��ƿ�У��ѹ۲쵽����ͼ������ݣ������±���
| ����ƿ��� | 1 | 2 | 3 | 4 | 5 |
| ����ƿ��������������� | 28.9% | 36.8% | 44.7% | 52.6% | 60.5% |
| ������ľ����״�� | �� | �� | ���� | ��ȼ | ��ȼ |
��1��ʵ�����в��÷ֽ�������⣨H2O2���ķ�����ȡ�������䷴Ӧ�Ļ�ѧ����ʽ��______ 2H2O+O2��
��2008?���ݣ�ijУ�о�С���ͬѧ����������ʵ�飺
������1����5�������ͬ�����Ͽ�Ȫˮƿ�ֱ��ռ�1ƿ������1ƿCO2�Լ�3ƿ������CO2�Ļ�����壨CO2�����ֱ�Ϊ10%��20%��30%�������ô��¶ȼƵĽ�������ƿ�ڣ�
������2��������װ������Ŀ�Ȫˮƿ�ŵ��׳�������䣬ÿ��1���Ӽ�¼һ���¶ȣ�ʵ�����ݼ�¼���ұ���ʾ��������ұ����ش��������⣺
��1������ʵ���Ŀ���ǣ�______��
��2�����ݱ��λ���о�Ŀ�ģ���ʵ����Եó��Ľ����У�______��
��3�����ݱ��λ�����������Ʋ⣬����ͬ�����£���CO2����Ϊ25%ʱ���¶ȴﵽ25.4����Ҫ��ʱ�䷶ΧΪ______min��
������1����5�������ͬ�����Ͽ�Ȫˮƿ�ֱ��ռ�1ƿ������1ƿCO2�Լ�3ƿ������CO2�Ļ�����壨CO2�����ֱ�Ϊ10%��20%��30%�������ô��¶ȼƵĽ�������ƿ�ڣ�
������2��������װ������Ŀ�Ȫˮƿ�ŵ��׳�������䣬ÿ��1���Ӽ�¼һ���¶ȣ�ʵ�����ݼ�¼���ұ���ʾ��������ұ����ش��������⣺
| ʱ��/min �¶�/�� CO2���� | ��ʼ | 1 | 2 | 3 | 4 | 5 |
| ȫ������ | 23.0 | 23.3 | 23.9 | 24.6 | 25.0 | 25.5 |
| 10% | 23.0 | 23.5 | 24.2 | 25.0 | 26.0 | 27.0 |
| 20% | 23.0 | 24.0 | 24.5 | 25.6 | 26.5 | 27.5 |
| 30% | 23.0 | 24.5 | 25.0 | 26.2 | 27.5 | 29.0 |
| 100% | 23.0 | 30.0 | 33.0 | 35.6 | 37.0 | 39.5 |
��2�����ݱ��λ���о�Ŀ�ģ���ʵ����Եó��Ľ����У�______��
��3�����ݱ��λ�����������Ʋ⣬����ͬ�����£���CO2����Ϊ25%ʱ���¶ȴﵽ25.4����Ҫ��ʱ�䷶ΧΪ______min��
��2008?���ݣ�ijУ�о�С���ͬѧ����������ʵ�飺
������1����5�������ͬ�����Ͽ�Ȫˮƿ�ֱ��ռ�1ƿ������1ƿCO2�Լ�3ƿ������CO2�Ļ�����壨CO2�����ֱ�Ϊ10%��20%��30%�������ô��¶ȼƵĽ�������ƿ�ڣ�
������2��������װ������Ŀ�Ȫˮƿ�ŵ��׳�������䣬ÿ��1���Ӽ�¼һ���¶ȣ�ʵ�����ݼ�¼���ұ���ʾ��������ұ����ش��������⣺
��1������ʵ���Ŀ���ǣ�______��
��2�����ݱ��λ���о�Ŀ�ģ���ʵ����Եó��Ľ����У�______��
��3�����ݱ��λ�����������Ʋ⣬����ͬ�����£���CO2����Ϊ25%ʱ���¶ȴﵽ25.4����Ҫ��ʱ�䷶ΧΪ______min��
������1����5�������ͬ�����Ͽ�Ȫˮƿ�ֱ��ռ�1ƿ������1ƿCO2�Լ�3ƿ������CO2�Ļ�����壨CO2�����ֱ�Ϊ10%��20%��30%�������ô��¶ȼƵĽ�������ƿ�ڣ�
������2��������װ������Ŀ�Ȫˮƿ�ŵ��׳�������䣬ÿ��1���Ӽ�¼һ���¶ȣ�ʵ�����ݼ�¼���ұ���ʾ��������ұ����ش��������⣺
| ʱ��/min �¶�/�� CO2���� | ��ʼ | 1 | 2 | 3 | 4 | 5 |
| ȫ������ | 23.0 | 23.3 | 23.9 | 24.6 | 25.0 | 25.5 |
| 10% | 23.0 | 23.5 | 24.2 | 25.0 | 26.0 | 27.0 |
| 20% | 23.0 | 24.0 | 24.5 | 25.6 | 26.5 | 27.5 |
| 30% | 23.0 | 24.5 | 25.0 | 26.2 | 27.5 | 29.0 |
| 100% | 23.0 | 30.0 | 33.0 | 35.6 | 37.0 | 39.5 |
��2�����ݱ��λ���о�Ŀ�ģ���ʵ����Եó��Ľ����У�______��
��3�����ݱ��λ�����������Ʋ⣬����ͬ�����£���CO2����Ϊ25%ʱ���¶ȴﵽ25.4����Ҫ��ʱ�䷶ΧΪ______min��