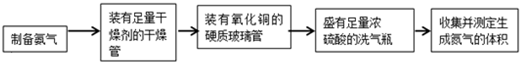
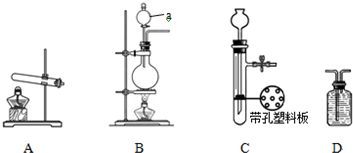
��Ŀ����
��ش��������� �����У�1����2����С������ѡһ��ش�����С�ⶼ�ش����Եڣ�1��С��Ĵ�����������
�������Ǹ��õط����������������ε������ںţ�Ϊ��������������Ϊ�����������ζȼٵ��������ǡ�����Ϊһ����������Ӧ�û�����ע��չ�뻷��֮��Ĺ�ϵ������ֱ�ӱ���ˮ��Դ�ͱ��������ĽǶ���ȫ�������������飺
��1���ӱ���ˮ��Դ�ĽǶȣ�
��2���ӱ��������ĽǶȣ�
�⣺��1���ӱ���ˮ��Դ�ĽǶȣ����ú���ϴ�·ۡ�������ˮ����ҵ��ˮ�����������ŷŵȶ����ԣ�
��2���ӱ��������ĽǶȣ���ֹ��ũ������սոˡ�������װβ������װ�õȶ����ԣ�
�ʴ�Ϊ����1���ӱ���ˮ��Դ�ĽǶȣ��ٽ��ú���ϴ�·ۣ���������ˮ����ҵ��ˮ�����������ŷţ�
��2���ӱ��������ĽǶȣ��ٽ�ֹ��ũ������սոˣ���������װβ������װ�ã�
������Ϊ�˴����÷�չ�뻷��֮��Ĺ�ϵ�����Ǵӱ���ˮ��Դ�ĽǶȣ����ú���ϴ�·ۡ�������ˮ����ҵ��ˮ�����������ŷŵȶ����ԣ��ӱ��������ĽǶȣ���ֹ��ũ������սոˡ�������װβ������װ�õȶ����ԣ�
���������⿼���ֹ��Ⱦ������ˮ��Դ�Ϳ�����������װβ������װ�õȶ����Ա���������
��2���ӱ��������ĽǶȣ���ֹ��ũ������սոˡ�������װβ������װ�õȶ����ԣ�
�ʴ�Ϊ����1���ӱ���ˮ��Դ�ĽǶȣ��ٽ��ú���ϴ�·ۣ���������ˮ����ҵ��ˮ�����������ŷţ�
��2���ӱ��������ĽǶȣ��ٽ�ֹ��ũ������սոˣ���������װβ������װ�ã�
������Ϊ�˴����÷�չ�뻷��֮��Ĺ�ϵ�����Ǵӱ���ˮ��Դ�ĽǶȣ����ú���ϴ�·ۡ�������ˮ����ҵ��ˮ�����������ŷŵȶ����ԣ��ӱ��������ĽǶȣ���ֹ��ũ������սոˡ�������װβ������װ�õȶ����ԣ�
���������⿼���ֹ��Ⱦ������ˮ��Դ�Ϳ�����������װβ������װ�õȶ����Ա���������

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
�Ҵ���C2H5OH�����Ը��������ס������Ϊԭ�ϣ��������͡������Ƶã����ڿ�������Դ���Ҵ�����������������ȫȼ��ʱ���ɶ�����̼��ˮ������������㣬�Ҵ�ȼ�տ��ܻ���һ����̼���ɣ�������ͼʵ��װ�ý���ʵ�飬�����Ҵ���ȼ�ղ������Ƿ��ж�����̼��һ����̼����������ʾ��CO+CuO
CO2+Cu��

��ش��������⣺
��1��д���Ҵ�����������������ȫȼ�յĻ�ѧ����ʽ ��
��2���мס��ҡ�������ͬѧ�ֱ��������ʵ�鲢���۲쵽��������д���±��У����á��С�����û�С��͡������С���д�±��е�ʵ����ۣ�
��3����ȼ��һ�������Ҵ����õ���4.4g������̼��5.6gһ����̼���ڸ÷�Ӧ����������ˮ�������� ��
| ||

��ش��������⣺
��1��д���Ҵ�����������������ȫȼ�յĻ�ѧ����ʽ
��2���мס��ҡ�������ͬѧ�ֱ��������ʵ�鲢���۲쵽��������д���±��У����á��С�����û�С��͡������С���д�±��е�ʵ����ۣ�
| ʵ������ | ʵ����� | ||||
| Aװ�� | Cװ�� | Eװ�� | �Ƿ��ж�����̼ | �Ƿ���һ����̼ | |
| ��ͬѧ | ʯ��ˮ ����� |
ʯ��ˮ û����� |
ʯ��ˮ û����� |
||
| ��ͬѧ | ʯ��ˮ ����� |
ʯ��ˮ ����� |
ʯ��ˮ ����� |
||
| ��ͬѧ | ʯ��ˮ û����� |
ʯ��ˮ û����� |
ʯ��ˮ ����� |
||
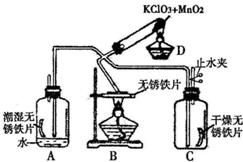 33��Ϊ����֤���������ڲ�ͬ�����µķ�Ӧ����������ͼ��ʾ����������̨����ȥ����ʵ�飬Dװ����O2����װ�ã�AΪ������ˮ��������O2��Ӧװ�ã�BΪ������Ƭ�ڼ�����������O2��Ӧװ�ã�Cװ������֤�ڸ���������O2������Ӧװ�ã�Cװ�����ȸ����������ش��������⣺
33��Ϊ����֤���������ڲ�ͬ�����µķ�Ӧ����������ͼ��ʾ����������̨����ȥ����ʵ�飬Dװ����O2����װ�ã�AΪ������ˮ��������O2��Ӧװ�ã�BΪ������Ƭ�ڼ�����������O2��Ӧװ�ã�Cװ������֤�ڸ���������O2������Ӧװ�ã�Cװ�����ȸ����������ش��������⣺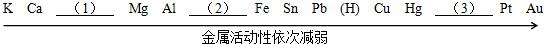
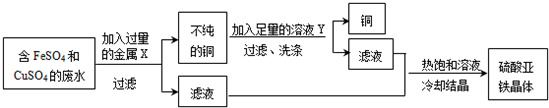
 ��2013?���ţ�ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij����ͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���14.0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У���ش��������⣺
��2013?���ţ�ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij����ͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���14.0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У���ش��������⣺