��Ŀ����
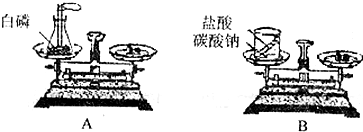 ij��ѧС��ͬѧ����ͼ��ʾʵ����֤�����غ㶨�ɣ�
ij��ѧС��ͬѧ����ͼ��ʾʵ����֤�����غ㶨�ɣ���1��д��Aͼ�з�����Ӧ�Ļ�ѧ����ʽ
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
| ||
��2��Bͼ��̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ�ǣ�
Na2CO3+2HCl�T2NaCl+CO2��+X������X�Ļ�ѧʽΪ
H2O
H2O
��3��С��ͬѧ��ʵ��۲쵽Aʵ�鷴Ӧǰ���������䣬Bʵ�鷴Ӧ�����������ˣ�������ͬѧ��Ϊ���������еķ�Ӧ�����������غ㶨�ɣ�����Ϊ���ǵĹ۵��Ƿ���ȷ
����ȷ
����ȷ
��Bʵ���������ٵ�ԭ��������������ɢ�ݵ�������
����������ɢ�ݵ�������
��4����Bʵ���У�ʵ��ǰ��ƽʾ��Ϊ102.2g��ʵ�����ƽʾ��Ϊ100g�������ɶ�����̼����Ϊ
2.2
2.2
g����5����������ʵ��ͷ����ܽ������֤�����غ㶨��ʵ��ʱ��Ҫע���������
������μӻ����ɵķ�ӦҪ���ܱյ������н���
������μӻ����ɵķ�ӦҪ���ܱյ������н���
����6��������ʵ���֪����ѧ��Ӧǰ��һ���������
C
C
������ţ���ԭ�������ԭ����Ŀ�۷�������ܷ�����Ŀ��Ԫ�����������������
A���٢ڢۢ�B���٢ڢܢ�C���٢ڢݢ�D���٢ڢܢ�
��������1�����ݷ�Ӧ���Ӧ�������������д��ѧ����ʽ��
��2�����������غ㶨�ɵ�Ԫ���غ㣬�Ʋ�����X�Ļ�ѧʽ��
��3�����еĻ�ѧ��Ӧ�����������غ㶨�ɣ������⿼��������ݳ���
��4��������֪���ݣ��Ʋ����ɵĶ�����̼��������
��5���˽�ʵ���ע�����
��6�����������غ㶨�ɵĺ�ۺ��ۺ��忼�ǣ�
��2�����������غ㶨�ɵ�Ԫ���غ㣬�Ʋ�����X�Ļ�ѧʽ��
��3�����еĻ�ѧ��Ӧ�����������غ㶨�ɣ������⿼��������ݳ���
��4��������֪���ݣ��Ʋ����ɵĶ�����̼��������
��5���˽�ʵ���ע�����
��6�����������غ㶨�ɵĺ�ۺ��ۺ��忼�ǣ�
����⣺��1��Aͼ�а���ȼ�շ�����Ӧ�Ļ�ѧ����ʽΪ 4P+5O2
2P2O5
��2��Bͼ��̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ�ǣ�Na2CO3+2HCl�T2NaCl+CO2��+X�����������غ㶨�ɣ�ԭ�ӵ������������Ӧǰ�䣬������X�Ļ�ѧʽΪ H2O
��3��С��ͬѧ��ʵ��۲쵽Aʵ�鷴Ӧǰ���������䣬Bʵ�鷴Ӧ�����������ˣ�������ͬѧ��Ϊ���������еķ�Ӧ�����������غ㶨�ɣ����ǵĹ۵� ����ȷ��Bʵ���������ٵ�ԭ���� ����������ɢ�ݵ�������
��4����Bʵ���У�ʵ��ǰ��ƽʾ��Ϊ102.2g��ʵ�����ƽʾ��Ϊ100g�������ɶ�����̼����Ϊ102.2g-100g=2.2g��
��5����������ʵ��ͷ����ܽ������֤�����غ㶨��ʵ��ʱ��Ҫע��������� ������μӻ����ɵķ�ӦҪ���ܱյ������н��У�
��6��������ʵ���֪����ѧ��Ӧǰ��һ��������У�ԭ�����ࡢԭ����Ŀ��Ԫ�����ࡢ������������
�ʴ�Ϊ����1��4P+5O2
2P2O5����2��H2O����3������ȷ�� ����������ɢ�ݵ������У���4��2.2
��5��������μӻ����ɵķ�ӦҪ���ܱյ������н��У���Ҫ���ܱյ������н��У�����Bװ��Ӧѡ��û������μӻ����ɵķ�Ӧ�Ⱥ������ɣ����ܴ�Ҫ��û���������ɵ�ҩƷ����˼�Ĵ𰸣�
��6��C
| ||
��2��Bͼ��̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ�ǣ�Na2CO3+2HCl�T2NaCl+CO2��+X�����������غ㶨�ɣ�ԭ�ӵ������������Ӧǰ�䣬������X�Ļ�ѧʽΪ H2O
��3��С��ͬѧ��ʵ��۲쵽Aʵ�鷴Ӧǰ���������䣬Bʵ�鷴Ӧ�����������ˣ�������ͬѧ��Ϊ���������еķ�Ӧ�����������غ㶨�ɣ����ǵĹ۵� ����ȷ��Bʵ���������ٵ�ԭ���� ����������ɢ�ݵ�������
��4����Bʵ���У�ʵ��ǰ��ƽʾ��Ϊ102.2g��ʵ�����ƽʾ��Ϊ100g�������ɶ�����̼����Ϊ102.2g-100g=2.2g��
��5����������ʵ��ͷ����ܽ������֤�����غ㶨��ʵ��ʱ��Ҫע��������� ������μӻ����ɵķ�ӦҪ���ܱյ������н��У�
��6��������ʵ���֪����ѧ��Ӧǰ��һ��������У�ԭ�����ࡢԭ����Ŀ��Ԫ�����ࡢ������������
�ʴ�Ϊ����1��4P+5O2
| ||
��5��������μӻ����ɵķ�ӦҪ���ܱյ������н��У���Ҫ���ܱյ������н��У�����Bװ��Ӧѡ��û������μӻ����ɵķ�Ӧ�Ⱥ������ɣ����ܴ�Ҫ��û���������ɵ�ҩƷ����˼�Ĵ𰸣�
��6��C
���������⿼����������غ㶨�ɵ����ã����������Ŀ���������������غ㶨�ɵĸ�����ա��μӡ����������ܺ͡��ĺ��壬��ȷ�����غ㶨�ɵ��۽��ͣ���ǻ�ѧ��Ӧǰ���������䡢����һ���䡢һ�����ܱ䣬Ȼ�������Բ�ͬ��Ŀ����IJ�ͬ���ص�������Ӧ֪ʶ���֢��ҩ��

��ϰ��ϵ�д�
�����Ŀ
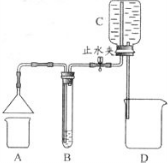 ��ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������2����Ȥʵ�飮ÿ��ʵ��ʱ����ֹˮ�У�Cƿ�ڵ�ˮ����D�У�ͬʱ����B���������ݳ���
��ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������2����Ȥʵ�飮ÿ��ʵ��ʱ����ֹˮ�У�Cƿ�ڵ�ˮ����D�У�ͬʱ����B���������ݳ���
 ��2013?������ģ����ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������Ȥʵ�飮
��2013?������ģ����ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������Ȥʵ�飮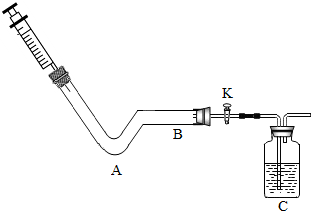 ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮
ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮