��Ŀ����
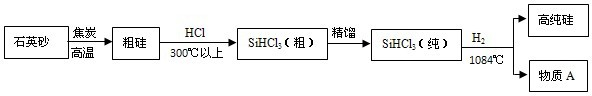
��Ϊ���С���̼���á�����Ҫ�Ƽ�������̫���� ��������Ϊ2010���Ϻ�������ġ����㡱��̫���ܹ��������ؼ��IJ����Ǹߴ��衣���ȼ��飨SiHCl3����ԭ���ǵ�ǰ�Ʊ��ߴ������Ҫ��������������ʾ��ͼ���£�

��1�����ȼ��飨SiHCl3������ ��Ԫ����ɵĻ���
��2��������Ҳ�������һ����ʽ��ͨ������ɰ�Һ��������뿪��ԭ�������û������ɷֵ�
����۵㡱�е㡱����ͬ��
��3��д���Ӵֹ赽SiHCl3���֣��Ļ�ѧ����ʽ ��
��4��Ϊ�˴ﵽ��ɫ��ѧ����Դ�ۺ����õ�Ŀ�ģ���������������ijЩ���ʿ�ѭ��ʹ�ã���Щ������
���ѧʽ����
��5��ʯӢɰ����Ҫ�ɷ���SiO2����������Al2O3����Ҫ��ȥʯӢɰ�����������ʣ���ѡ�õ��Լ���
���ѧʽ����
��2��������Ҳ�������һ����ʽ��ͨ������ɰ�Һ��������뿪��ԭ�������û������ɷֵ�
����۵㡱�е㡱����ͬ��
��3��д���Ӵֹ赽SiHCl3���֣��Ļ�ѧ����ʽ ��
��4��Ϊ�˴ﵽ��ɫ��ѧ����Դ�ۺ����õ�Ŀ�ģ���������������ijЩ���ʿ�ѭ��ʹ�ã���Щ������
���ѧʽ����
��5��ʯӢɰ����Ҫ�ɷ���SiO2����������Al2O3����Ҫ��ȥʯӢɰ�����������ʣ���ѡ�õ��Լ���
���ѧʽ����
��1��3
��2���е�
��3��Si+3HCl SiHCl3+H2
SiHCl3+H2
��4��H2��HCl
��5��HCl��HNO3��H2SO4
��2���е�
��3��Si+3HCl
 SiHCl3+H2
SiHCl3+H2 ��4��H2��HCl
��5��HCl��HNO3��H2SO4

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ




 Si+2R��R�Ļ�ѧʽΪ______���÷�Ӧ�Ļ���������____________��
Si+2R��R�Ļ�ѧʽΪ______���÷�Ӧ�Ļ���������____________��