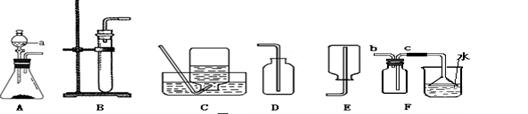
��Ŀ����
���������ʵ��װ��ͼ�ش����⡣
��1��д������a��b�����ƣ�a�� ��b�� ��
��2������Bװ����ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ�����ü����Ȼ�狀��������ƹ�������ķ�����ȡ������NH3����ͬʱ�õ��� ���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ�� ��Ӧѡ�õķ���װ��Ϊ ����װ�ñ�ţ���
��4���ռ�����ʱӦѡ��Dװ�ã����ռ��������ļ���ƿ�����ڵ�����ɫ��̪��Һ��
ˮ���У��۲쵽����ƿ���д�����ɫҺ����롣����������Ϣ�ܽ������������
�� �� ���ش��������ɣ���
��1���Թ� ��Һ©��
��2��2H2O2 2H2O+O2��
2H2O+O2��
��3��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O A
CaCl2+2NH3��+2H2O A
��4���������ܶȱȿ���С ������������ˮ�γɰ�ˮ
���������������1��������������ͼ��a���Թܣ�b�Ƿ�Һ©�����ʴ�Ϊ���Թܣ���Һ©����
��2��Bװ�������ڹ����Һ��IJ����ȷ�Ӧ����ȡ����ʱ���ö��������������ֽ����������Һ����ѧ��Ӧ����ʽ�ǣ�2H2O2 2H2O+O2����
2H2O+O2����
��3���Ƶð������ü����Ȼ�狀��������ƹ�������ķ��������ڡ���������͡�������ѡ��A����ѧ��Ӧ����ʽ�ǣ�2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��4���ռ�����ʱӦѡ��Dװ�ã�˵���������ܶ�С�ڿ������ܶȣ���ˮ�Լ��ԣ�������Һ��ʹ��̪��Һ��죬����ƿ���д�����ɫҺ����룬˵��������������ˮ�γɰ�ˮ���ʴ�Ϊ���������ܶȱȿ���С��������������ˮ�γɰ�ˮ��
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ����������������ռ�����

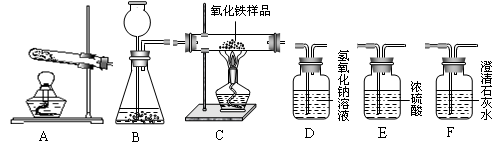
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�ij����ֻ���������ſ������ռ������������е����������ǣ� ��
| A��������ˮ | B����������ˮ |
| C������������еijɷַ�����Ӧ | D�� |
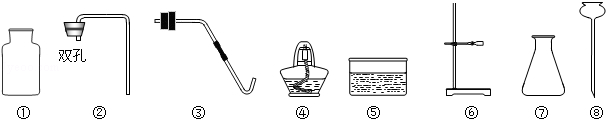
��ͼ��һЩ����ķ���װ�ú��ռ�װ�ã��밴Ҫ��ش�
��1��д������������������ƣ��� ���� ���� ��
��2����ʵ������KMnO4Ϊԭ����ȡ���ռ�һƿO2��Ӧѡ�� (��д���)װ�ý�����װ��
��3���Ƚ��ù���������Һ���ø��������ȡO2�ķ��������ߵĹ�ͬ���� ����д��ţ���
| A������װ����ͬ |
| B��������MnO2������ |
| C����Ӧ�Ļ���������ͬ |
| D����ȫ��Ӧ��ʣ�����ɷ���ͬ |
��5������ͬѧ�����ʵ������ȡCO2װ�ã���ͼ��ʾ����װ���г�����һ��������ľ��������� ���ܷ�����˿��������ͭ˿�� ���� ���ܡ��� �����ܡ��������� ����װ����ͨ�� ���Ʒ�Ӧ��ʼ�������