��Ŀ����
�ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㡣�ס��ҡ���������ʾ�������ʣ����ǵIJ��ֻ�ѧʽ����ʾ��ͼ�ֱ������±���
|
���� |
�� |
�� |
�� |
�� |
|
|
��ѧʽ |
? |
C |
CO |
H2 |
|
|
�� ʾ��ͼ |
|
|
|
�� |
��1��д�������ʵĻ�ѧʽ �����������ʵ���ʾ��ͼ ��
��2�����ɶ����ʵ������� ������ӡ���ԭ�ӡ������ӡ�����
����������������̬�ǽ������ʵ��� ���ѧʽ����ͬ����
�ж��Ե��� ��
��3�������£����ҷ�Ӧ���ɱ��Ͷ����÷�Ӧ�Ļ�ѧ����ʽΪ
��
��1��H2O�� ��2�����ӣ�
H2�� CO ��3��C+H2O
��2�����ӣ�
H2�� CO ��3��C+H2O CO+H2
CO+H2
��������
����������������֪��
��1�������ʵĻ�ѧʽΪH2O�������ʵ���ʾ��ͼ�� ��
��
��2�����ɶ����ʵ������Ƿ��ӣ�����������������̬�ǽ������ʵ���H2���ж��Ե���CO��
��3�������£����ҷ�Ӧ���ɱ��Ͷ����÷�Ӧ�Ļ�ѧ����ʽΪC+H2O CO+H2��
CO+H2��
���㣺���ʵĹ��ɣ���ѧ����ʽ��һ����̼�����ʡ�
�����������Ѷ�һ�㣬�ؼ��Ƕ������⣬����ͼʾ�ó����ʵķ��ӹ��ɣ�����д����ѧʽ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ���� | A | B | C | D |  --��ԭ�� --��ԭ�� --̼ԭ�� --̼ԭ�� --��ԭ�� --��ԭ�� |
| ��ʾ��ͼ |  |
 |
 |
 |
��2���������������ڵ��ʵ���
��3��C����������Ԫ�ص�������Ϊ
��4����һ��������A��B��C��D�������ʷ���һ�ܱյ��������У��ڵ�������£�������ַ�Ӧ����÷�Ӧǰ������ʵ��������£�
| A | B | C | D | |
| ��Ӧǰ����/g | 25 | 1 | 10 | 74 |
| ��Ӧ������/g | 9 | 37 | a | 10 |





 ������
������ ������
������ ���ֱ��ʾA��B��C�������ʵķ��ӣ���ͼ��ʾij��ѧ��Ӧǰ��Ӧ������������Ӽ�����Ŀ�ı仯���û�ѧ����ʽ��A��B��Cǰ�Ļ�ѧ������֮��Ϊ
���ֱ��ʾA��B��C�������ʵķ��ӣ���ͼ��ʾij��ѧ��Ӧǰ��Ӧ������������Ӽ�����Ŀ�ı仯���û�ѧ����ʽ��A��B��Cǰ�Ļ�ѧ������֮��Ϊ

 ��ʾAԪ�ص�ԭ�ӣ�
��ʾAԪ�ص�ԭ�ӣ� ��ʾBԪ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�
��ʾBԪ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�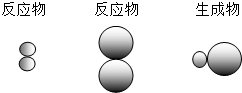
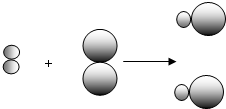 ��
��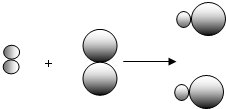 �е�
�е� ����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��
����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ�� ��ʾ��Ԫ�ص�ԭ�ӣ�
��ʾ��Ԫ�ص�ԭ�ӣ� ��ʾ��Ԫ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�
��ʾ��Ԫ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�


 ����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��
����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��




