��Ŀ����
14����Դ�ͻ����Ѿ���Ϊ���������ע�����⣮��1��Ŀǰ��������������Դ��ú��ʯ�ͺ���Ȼ���Ȼ�ʯȼ�ϣ�úȼ�ջ���������Ķ�����̼�����������һ����̼�����壬��Щ�����У�����ˮ�ᵼ���������Ҫ��SO2���ѧʽ����
��2��δ������Դ������ʵ������Ⱦ���ڴ������������£�ˮ�ֽ�������Դ��д���÷����������Դ�Ļ�ѧ����ʽ��2H2O$\frac{\underline{\;����\;}}{��}$2H2��+O2����ˮ�����ɻ������Դ����������Ҳ����Ҫ���ã�ˮ���ڣ�����ڡ������ڡ�������������������Ӫ����֮һ�����н϶�����Ըơ�þ�������ˮ����Ӳˮ�����ˮ����Ӳˮ��������֪ˮ���ܶ�Ϊ1.0g/mL��ʵ��������50g������������Ϊ5%���Ȼ�����Һ����Ҫ��ȡ�Ȼ���2.5g����Ҫ��ȡˮ47.5mL��
��3��COҲ������Դ���ʣ�ij��ѧ��ȤС���ͬѧ��һ���������������ۻ�϶��ɵĹ�����Ʒ����̽�������dz�ȡ��13.6g������Ʒ����ͼ1��ʾ��װ�ý���ʵ�飬�ⶨ�IJ���������ͼ2��ʾ��
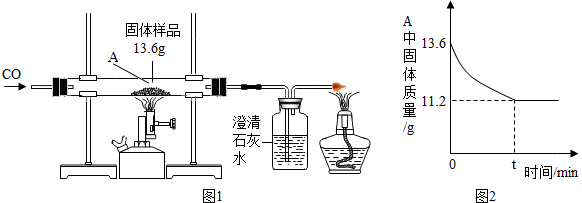
�ٴӻ��������ĽǶȣ��������һ����ʵ��Ľ��Ĵ�ʩ����β������װ�ã�
����������й����ݣ�������Ʒ���������������������������һλС�������ڴ���ֽ��д�������Ľ��ⲽ�裩��
���� ���ݻ�ѧ����Դ��֪ʶ������Դ�Ļ�ȡ����ѧ�����彡����֪ʶ�Լ�һ����̼��ԭ��������֪ʶ���з�����ɣ�
��� �⣺��1��Ŀǰ��������������Դ��ú��ʯ�ͺ���Ȼ���Ȼ�ʯȼ�ϣ�������̼�����������һ����̼�������У�����ˮ�ᵼ���������Ҫ�Ƕ��������ʯ�ͣ�SO2��
��2���ڴ������������£�ˮ�ֽ���������������ˮ�����ɻ������Դ��ˮ��������������������Ӫ����֮һ�����н϶�����Ըơ�þ�������ˮ����Ӳˮ��ʵ��������50g������������Ϊ5%���Ȼ�����Һ����Ҫ��ȡ�Ȼ���2.5g����ˮ������Ϊ��50g-2.5g=47.5g����Ҫ��ȡˮ$\frac{47.5g}{1g/mL}$=47.5mL�����2H2O$\frac{\underline{\;����\;}}{��}$2H2��+O2�������ڣ�Ӳˮ��47.5��
��3����һ����̼���ж������壬�ŷŵ������л���Ⱦ�������ʴӻ��������ĽǶȣ���Ҫ����β������װ�ã��������β������װ�ã�
���������Ʒ��������������Ϊx��
Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2����m
160 112 160-112=48
x 13.6g-11.2g=2.4g
$\frac{160}{x}=\frac{48}{2.4g}$
x=8g��
��Ʒ������������������=$\frac{8g}{13.6g}��100%$��58.8%
�𣺹�����Ʒ��������������������58.8%
���� ���⿼����ǻ�ѧ�������Լ����ݻ�ѧ����ʽ�ļ����֪ʶ����ɴ��⣬�����������е�֪ʶ���У�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �۲���ɫ | B�� | NaCl��Һ | C�� | ��̪��Һ | D�� | Ca��OH��2��Һ |
| A�� | ��֬�����ࡢ�����ʶ�����������Ӫ���أ������Խ��Խ�� | |
| B�� | Ϊ��ֹˮ��Ⱦ��Ӧ��ֹʹ�û��ʺ�ũҩ | |
| C�� | ��ˮ�����߲˿��Բ���ά���� | |
| D�� | ��ϴ�ྫȥ��������Ϊϴ�ྫ���ܽ����� |
| A�� | +2 | B�� | +3 | C�� | +4 | D�� | +5 |
| ѡ�� | ���ʣ�������Ϊ���ʣ� | ��ȥ���ʵķ��� |
| A | NaOH��Һ��Na2SO4�� | ����������Ba��OH��2��Һ������ |
| B | CaO��CaCO3�� | ���¼����������������� |
| C | CO2��CO�� | ͨ������������ȼ |
| D | CuSO4��Һ��H2SO4�� | ����������ͭ��ĩ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ȼ�ն��·��Ļ����¶Ȳ��״ﵽ�Ż�� | |
| B�� | �������Ż�������� | |
| C�� | ȱ����ȼ�� | |
| D�� | ȱ������ |
| A�� | ʯ�ͷ����Ƶ�ʯ�������� | B�� | ������ȥ�·��ϵ����� | ||
| C�� | �������м�����麟��ֳ��� | D�� | ��������ˮ����ɫ |