��Ŀ����
�����ͼʾ�ش��������⣺

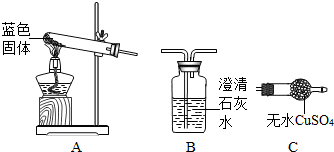
ij��ѧС��ͬѧ�����������ȡ��������֤�����װ�ã���ͼ������Һ©�������ų�һ��������������Һ���ϻ�����A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½���ϡ��������D�У��뿴ͼ�ش��������⣺
��1��B�а����ܹ���ˮ��ȼ�յ�ԭ����
��3����Fװ���ռ������������

ij��ѧС��ͬѧ�����������ȡ��������֤�����װ�ã���ͼ������Һ©�������ų�һ��������������Һ���ϻ�����A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½���ϡ��������D�У��뿴ͼ�ش��������⣺
��1��B�а����ܹ���ˮ��ȼ�յ�ԭ����
�ṩ������
�ṩ������
����2��E�е�ʵ����������Һ���
��Һ���
����3����Fװ���ռ������������
���ܶȴ��ڿ������ܶ�
���ܶȴ��ڿ������ܶ�
����4��D�з�Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
���������������е�֪ʶ���з�����ȼ����Ҫ��ȼ���������Ӵ����¶ȴﵽ��ȼ����Ż�㣬��ɫʯ����Һ��������Һ��죬F�ռ���������Ҫ�ܶȱȿ�����̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��
����⣺��1����������ֽ����ɵ���������B�У��ṩ��ȼ������Ҫ������������ṩ��������
��2��A�����ɵ�����ʹװ���ڵ�ѹǿ�����ܽ�����ѹ��D�У�ʹ������̼��Ʒ�Ӧ���ɶ�����̼���壬������̼����E�У�������̼��ˮ��Һ�����ԣ���ʹʯ����Һ��죬�����Һ��죻
��3��F�������ſ������ռ����壬��ʹ�ô˷��ռ���������Ҫ�߱��ܶȱȿ���������ʣ�������ܶȴ��ڿ������ܶȣ�
��4��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����CaCO3+2HCl�TCaCl2+H2O+CO2����
��2��A�����ɵ�����ʹװ���ڵ�ѹǿ�����ܽ�����ѹ��D�У�ʹ������̼��Ʒ�Ӧ���ɶ�����̼���壬������̼����E�У�������̼��ˮ��Һ�����ԣ���ʹʯ����Һ��죬�����Һ��죻
��3��F�������ſ������ռ����壬��ʹ�ô˷��ռ���������Ҫ�߱��ܶȱȿ���������ʣ�������ܶȴ��ڿ������ܶȣ�
��4��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����CaCO3+2HCl�TCaCl2+H2O+CO2����
���������⿼�����������ȡ����������ʣ���ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ