��Ŀ����
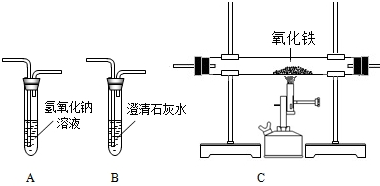
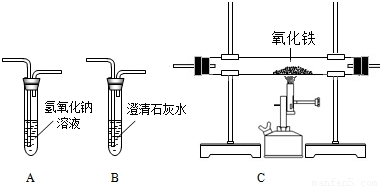
����ij�������ŷŵ�β���г���CO2�⣬�����ܺ���CO�����塣Ϊȷ��CO�����Ƿ���ڣ�ʵ��С����������װ�ý����˼��飬��ش��������⣺

��1����ʵ��ʱ������װ�õ�����˳���ǣ�A��B��C��B������Aװ�õ�������____________����һ��Bװ�õ�����Ϊ______________��
��2����CO������ڣ���װ��C��ʵ������Ϊ________________����Ӧ�Ļ�ѧ����ʽΪ___________��
��3���ӻ��������ĽǶȿ��ǣ�����Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�������_____________��
��2����CO������ڣ���װ��C��ʵ������Ϊ________________����Ӧ�Ļ�ѧ����ʽΪ___________��
��3���ӻ��������ĽǶȿ��ǣ�����Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�������_____________��
��1����ȥԭβ���еĶ�����̼���壻����ԭβ���еĶ�����̼�Ƿ������
��2����ɫ������ڣ�3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
��3������β������װ�ã���β����ȼ�����������ռ�β��
��2����ɫ������ڣ�3CO+Fe2O3
 2Fe+3CO2
2Fe+3CO2��3������β������װ�ã���β����ȼ�����������ռ�β��

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ