��Ŀ����
ˮ��Ӳ�ȱ�ʾ�����ǣ���ˮ�е�Ca2+��Mg2+���������CaO��������ͨ����1Lˮ�к���10mg CaO��Ϊ1�ȣ�1Lˮ�к���20mg CaO��Ϊ2�ȣ��Դ����ƣ�8������ΪӲˮ��8������Ϊ��ˮ���ҹ��涨����ˮ��Ӳ�Ȳ��ܳ���25�ȣ���1���ճ������У����ü���ijˮ����Ӳˮ������ˮ��
��2��Ca��HCO3��2����ʱ��ֽ����һ�ְ�ɫ������ˮ������Ҫ�ɷ֣������ֳ������������д��������Ӧ�Ļ�ѧ����ʽ����ˮ����ˮ������һ������Ca��HCO3��2������ǰ�ɲ�ȡ�ķ���������ˮ��Ӳ�ȣ�
��3��������ˮƿһ������ˮ�����䱣�����ܻή�ͣ���ϡ����ɳ�ȥ����ˮ����������Ӧ�Ļ�ѧ����ʽΪ��
��4��ȡijˮ��1L����ʵ��ⶨ�����к�Ca2+0.2g����ˮ����Ӳ��ԼΪ���Ƿ��������ˮ������
���𰸡�����������Ӳˮ����ˮ�ļ��鷽�����ɽ��1����������Ŀ������Ϣ���Խ��2����������о�ˮ��õ���������ˮ�Ĺ������Ҫ�ɷ���̼��ƣ���֪̼���εļ��鷽�����ɽ��3��������Ϣ���Լ����ˮ����Ӳ�Ȳ��жϳ��Ƿ���ϱ���
����⣺��ˮ��Ӳˮ������ˮ����ˮ�����÷���ˮ�������ǣ�����̼���εķ����Ǽ�����������ᣬ�����ǹ����ܽ⣬�����������0.2g=200mg���������Լ������CaO������Ϊ280mg���ʸ�ˮ����Ӳ��Ϊ28�ȣ�
�ʴ�Ϊ����1������ˮ
��2��Ca��HCO3��2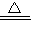 CaCO3��+H2O+CO2�� ���
CaCO3��+H2O+CO2�� ���
��3��CaCO3+2HCl�TCaCl2+H2O+CO2����Mg��OH��2+2HCl�TMgCl2+2H2O
��4��28 ������
���������⿼��Ӳˮ����ˮ�ļ��顢����Ϣ����������̼���εļ��鷽��������Ĺؼ������ռ��鷽���Ͷ�����Ϣ����������
����⣺��ˮ��Ӳˮ������ˮ����ˮ�����÷���ˮ�������ǣ�����̼���εķ����Ǽ�����������ᣬ�����ǹ����ܽ⣬�����������0.2g=200mg���������Լ������CaO������Ϊ280mg���ʸ�ˮ����Ӳ��Ϊ28�ȣ�
�ʴ�Ϊ����1������ˮ
��2��Ca��HCO3��2
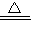 CaCO3��+H2O+CO2�� ���
CaCO3��+H2O+CO2�� �����3��CaCO3+2HCl�TCaCl2+H2O+CO2����Mg��OH��2+2HCl�TMgCl2+2H2O
��4��28 ������
���������⿼��Ӳˮ����ˮ�ļ��顢����Ϣ����������̼���εļ��鷽��������Ĺؼ������ռ��鷽���Ͷ�����Ϣ����������

��ϰ��ϵ�д�
�����Ŀ