��Ŀ����
��6�֣���ѧ��ȤС���ͬѧȡ10 gijп��Ʒ�����������ʣ������ʲ�����ˮ��Ҳ�����ᷴӦ�����ձ��У������м���һ������ϡ���ᣬ������ϡ���������Ϊ93��7 gʱ��ǡ����ȫ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺

��1����ͼ�п��Կ�������ȫ��Ӧ����������������Ϊ g��
��2����Ʒ��п������Ϊ���٣���3����Ӧ��������Һ������п����������Ϊ���٣�
���𰸡�
��6�֣�
��1��0��2��1�֣�
��2���⣺����Ʒ��п������Ϊx��
��Ӧ��������Һ������п������Ϊy
Zn+H2SO4=ZnSO4+H2�� ��1�֣�
65 161 2
x y 0.2 g ��1�֣�
65��x = 2�� 0.2 g x =6.5g ��1�֣�
��3��161��y = 2 �� 0.2 g y = 16.1g ��1�֣�
������Һ�У�����п����������Ϊ��
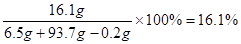 ��1�֣�
��1�֣�
����
����������

��ϰ��ϵ�д�
�����Ŀ
 ��ѧ��ȤС���ͬѧȡ10gijп��Ʒ�����������ʣ������ʲ�����ˮ��Ҳ�����ᷴӦ�����ձ��У������м���������ϡ���ᣬ��ȫ��Ӧ��������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺����ʾ��Zn+H2SO4�TZnSO4+H2����
��ѧ��ȤС���ͬѧȡ10gijп��Ʒ�����������ʣ������ʲ�����ˮ��Ҳ�����ᷴӦ�����ձ��У������м���������ϡ���ᣬ��ȫ��Ӧ��������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ���Իش��������⣺����ʾ��Zn+H2SO4�TZnSO4+H2���� ͭ���ڳ�ʪ�Ļ������ܹ�������ʴ����ͭ�⣬��Ҫ�ɷ��Ǽ�ʽ̼��ͭ��Ϊ���о���ʽ̼��ͭ�����Ԫ�أ�ij��ѧ��ȤС���ͬѧȡ�˲���ͭ�����Թ��и����������ȣ���ͼ��ʾ�������Թܱ���ˮ�飬˵����
ͭ���ڳ�ʪ�Ļ������ܹ�������ʴ����ͭ�⣬��Ҫ�ɷ��Ǽ�ʽ̼��ͭ��Ϊ���о���ʽ̼��ͭ�����Ԫ�أ�ij��ѧ��ȤС���ͬѧȡ�˲���ͭ�����Թ��и����������ȣ���ͼ��ʾ�������Թܱ���ˮ�飬˵����