��Ŀ����
��֪A��B��C���ֹ��庬��ͬ�ֽ���Ԫ�أ�A������ˮ��B��һ������������������D��E��F����ͬ�ַǽ���Ԫ�أ�E�dz����Ĺ��嵥�ʣ�D��Fͨ��״����Ϊ���塣X������Ԫ����ɣ�����EԪ�ص���������Ϊ37.5%������һ��Ԫ��ԭ�Ӹ�����Ϊ2��1�����ǵ�ת����ϵ����ͼ�����ֲ�������ȥ����

(1)д��E��X���ʵĻ�ѧʽ��E_________��X__________��
(2)д��C��A�Ļ�ѧ����ʽ��______________________________��
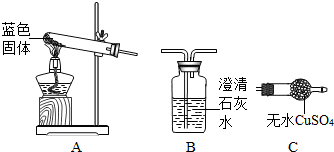
(3)ʵ������������D��F��ɵĻ�����壬�������ͼװ�ã���a��Ӧʢ��________����д�Լ����ƣ���ͬ����b��Ӧʢ��___________��
(2)д��C��A�Ļ�ѧ����ʽ��______________________________��
(3)ʵ������������D��F��ɵĻ�����壬�������ͼװ�ã���a��Ӧʢ��________����д�Լ����ƣ���ͬ����b��Ӧʢ��___________��
��1��C��CaC2
��2��Ca(OH)2+CO2==CaCO3��+H2O
��3��ŨNaOH��Һ��ϡH2SO4
��2��Ca(OH)2+CO2==CaCO3��+H2O
��3��ŨNaOH��Һ��ϡH2SO4

��ϰ��ϵ�д�
�����Ŀ
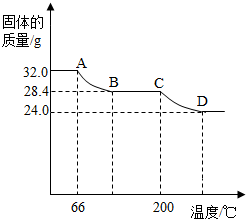

 ��ɹ��������仯��ֽ��¶ȵĹ�ϵ��ͼ��
��ɹ��������仯��ֽ��¶ȵĹ�ϵ��ͼ��



