��Ŀ����
ijУ��ѧ��ȤС��Ϊ�ⶨ�����ж�������ĺ�������
NaOH��Һ����SO2����Ӧ����ʽ���£� 2NaOH��SO2��Na2SO3��H2O��
NaOH��Һ����1000L�ѳ�ȥCO2�Ŀ�����Ʒ����Һ����������0.64g����֪��ʱ�������ܶ�ԼΪ1.3g/L����(1)�����յ�SO2������
(2)������Ӧ��NaOH��������
(3)������SO2������������(��������ȷ��0.01��)
�𰸣�
������
������
|
���� (1)��Һ����������0.64g���������յ�SO2���������� (2)�跢����Ӧ��NaOH������Ϊx�������� 2NaOH��SO2������ 80������64������ x��������0.64g���� ���� x��0.8g���� (3)������SO2������������ ������ (1)�����յ�SO2����Ϊ0.64g��(2)������Ӧ��NaOH������Ϊ0.8g��(3)������SO2����������Ϊ0.05���� |

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ



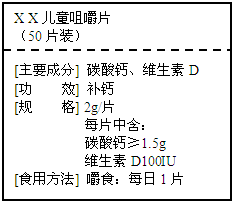
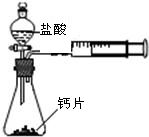
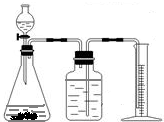
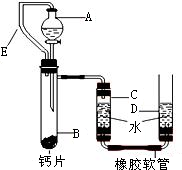
 +H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��
+H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��
