��Ŀ����
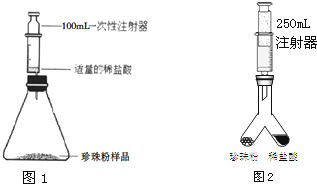
ͼ��1����С��ͬѧ��Ƶ�����ʵ������ȡ������װ�ã�
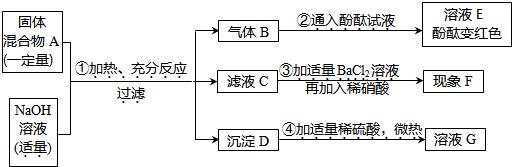
��1�����װ����ȣ���װ�õ��ŵ���______��
��2��������װ�ú�ҩƷ��ȡ������������Ӧ�Ļ�ѧ����ʽΪ��______�����ռ����������������ܵ�ԭ����______������ţ���
�ٵ��ܿ�һ�����ݾ������ռ�����װ��©�������ռ�������Թ���δװ��ˮ��
��3����������װ�õ��ռ�������Ϊ______��������ʵ������ȡ������̼���壬д��ʵ������ȡ������Ļ�ѧ����ʽ______��
��4�������ˮ��ͼ��2��װ������֤������̼������ˮ������������������______��
�⣺��1����װ�����г���©����������ʱ��������Һ����2����ȡ����ķ���ʽΪ��Zn+H2SO4=ZnSO4+H2���� ��������������ԭ��ࣺܶ�ٵ��ܿ�һ�����ݾ������ռ�����װ��©�������ռ�������Թ���δװ��ˮ����Щ���ܵ�����ȡ�������л��п�������3��������̼���ܶȱȿ����Ĵ��������ſ������ռ�����4����װ�ã�2������֤������̼������ˮ�IJ���������Ѹ�ٵ��뼸��ˮ�������Ǻò���Ƭ��ƿ���ã���������Ƭ������¼��ɣ�
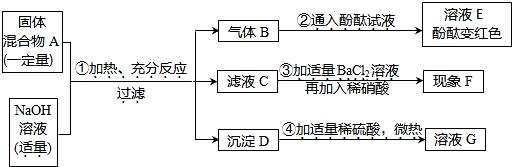
�ʴ�Ϊ����1����ʱ��������Һ��2��Zn+H2SO4=ZnSO4+H2���� �٢ڢ�
��3�������ſ����� CaCO3+2HCl=CaCl2+CO2��+H2O ��4��Ѹ�ٵ��뼸��ˮ�������Ǻò���Ƭ��ƿ���ã���������Ƭ�������
��������װ�����г���©����������ʱ��������Һ��������ܿ�һ�����ݾ������ռ���װ��©�����ռ�������Թ���δװ��ˮ����Щ���ܵ�����ȡ�������л��п�����ע�������̼���ܶȲ��������������ſ������ռ�����װ�ã�2������֤������̼�����ڣ�Ѹ�ٵ��뼸��ˮ�������Ǻò���Ƭ��ƿ���ã�������̼����ˮ��ƿ�ڵ�ѹ����С���ڴ���ѹ�������£���������Ƭ������£�
������ʵ������ȡ�����õ�����©��������ͨ������©����ʱ������Һ����ȷ��������������ԭ��
�ʴ�Ϊ����1����ʱ��������Һ��2��Zn+H2SO4=ZnSO4+H2���� �٢ڢ�
��3�������ſ����� CaCO3+2HCl=CaCl2+CO2��+H2O ��4��Ѹ�ٵ��뼸��ˮ�������Ǻò���Ƭ��ƿ���ã���������Ƭ�������
��������װ�����г���©����������ʱ��������Һ��������ܿ�һ�����ݾ������ռ���װ��©�����ռ�������Թ���δװ��ˮ����Щ���ܵ�����ȡ�������л��п�����ע�������̼���ܶȲ��������������ſ������ռ�����װ�ã�2������֤������̼�����ڣ�Ѹ�ٵ��뼸��ˮ�������Ǻò���Ƭ��ƿ���ã�������̼����ˮ��ƿ�ڵ�ѹ����С���ڴ���ѹ�������£���������Ƭ������£�
������ʵ������ȡ�����õ�����©��������ͨ������©����ʱ������Һ����ȷ��������������ԭ��

��ϰ��ϵ�д�
 ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�
�����Ŀ
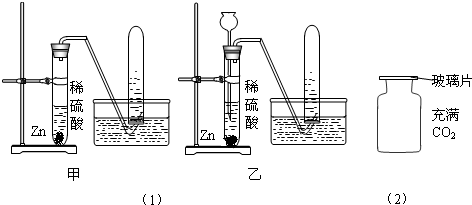
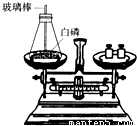
��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮
�ٰ�������ƿ��ȼ��ʱ����������ƿ�����忪��ʵ��ʧ�ܣ�����ƿ�����忪��ԭ����ʲô��
��Ϊ�˷�ֹƿ�����忪�����ܽ�ʵ������εĸĽ���
��ʵ��ʱ���ü��Ⱥ�IJ�������ȼ���ף��㻹���ҳ�����������ȼƿ�ڵİ�����
��2����֪��ҵ���õ���������ķ�����ȡ�������Ļ�ѧ����ʽΪ��2Al2O3 4Al+3O2�����ڽ�𣺡����10t�����������������ٶ�������һ�⣬
4Al+3O2�����ڽ�𣺡����10t�����������������ٶ�������һ�⣬
Сٻ��С����λͬѧ�ֱ�����������ֲ�ͬ�ⷨ��
��ش��������⣺
������Ϊ���ǵĽ���˼·�ͷ�������ȷ�𣿲�˵�����ɣ�
�ڶԡ�34g����������ȫ�ֽ������������Ϊ���ٿˣ���һ�⣬����ΪҲ�����������ַ�������𣿲�˵�����ɣ�
������Ѣڵ���ȷ�ⷨд������
�ٰ�������ƿ��ȼ��ʱ����������ƿ�����忪��ʵ��ʧ�ܣ�����ƿ�����忪��ԭ����ʲô��
��Ϊ�˷�ֹƿ�����忪�����ܽ�ʵ������εĸĽ���
��ʵ��ʱ���ü��Ⱥ�IJ�������ȼ���ף��㻹���ҳ�����������ȼƿ�ڵİ�����
��2����֪��ҵ���õ���������ķ�����ȡ�������Ļ�ѧ����ʽΪ��2Al2O3
 4Al+3O2�����ڽ�𣺡����10t�����������������ٶ�������һ�⣬
4Al+3O2�����ڽ�𣺡����10t�����������������ٶ�������һ�⣬Сٻ��С����λͬѧ�ֱ�����������ֲ�ͬ�ⷨ��
| Сٻͬѧ�Ľⷨ | С��ͬѧ�Ľⷨ |
| �⣺�����ɵ�������ΪX 2Al2O3  4Al+3O2�� 4Al+3O2��204108 10t X 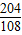 = =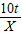 X=5.3t ����������5.3t�� | �⣺����������Ԫ�ص���������Ϊ 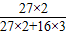 ×100%=53% ×100%=53%��������Ϊ10t×53%=5.3t ����������5.3t�� |
������Ϊ���ǵĽ���˼·�ͷ�������ȷ�𣿲�˵�����ɣ�
�ڶԡ�34g����������ȫ�ֽ������������Ϊ���ٿˣ���һ�⣬����ΪҲ�����������ַ�������𣿲�˵�����ɣ�
������Ѣڵ���ȷ�ⷨд������

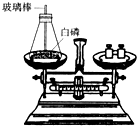 ��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮
��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮