��Ŀ����
��7�֣���2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ����ͼ��ʾ����ʾ��NaCl + AgNO3 = AgCl��+ NaNO3 ����

��1������A�㴦����NaNO3��������
��2������B����Һ��AgNO3����������������
�����������������������С�����һλ��
���𰸡�
��7�֣���1���⣺��A�㴦����NaNO3������Ϊx
AgNO3 + NaCl = AgCl�� + NaNO3
58.5 85
2.34g x
 =
=
x = 3.4g
��2����A�㴦��ӦAgNO3������Ϊy������AgCl������Ϊz
AgNO3 + NaCl = AgCl�� + NaNO3
170 58.5 143.5
y 2.34g z

m(B����Һ)=2.34g +103.4g + 200g - 5.74g = 300g
B����ҺAgNO3�������������� = �� 100% = 2.3%
�� 100% = 2.3%
���������������ո��֣�
��A�㴦����NaNO3������Ϊ3.4g��B����ҺAgNO3��������������Ϊ2.3%��
����������

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
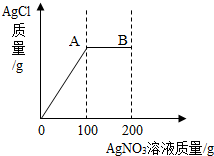 ��2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ��ͼ��ʾ����ʾ��NaCl+AgNO3=AgCl��+NaNO3 ����
��2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ��ͼ��ʾ����ʾ��NaCl+AgNO3=AgCl��+NaNO3 ����
 ��Һ��������ϵ����ͼ��ʾ����ʾ��NaCl + AgNO3 =" AgCl��+" NaNO3����
��Һ��������ϵ����ͼ��ʾ����ʾ��NaCl + AgNO3 =" AgCl��+" NaNO3����

