��Ŀ����
Ϊ�˲ⶨʯ��ʯ��Ʒ��̼��Ƶ������������ס�����ͬѧ�ֱ����������ʵ�飨���ʲ�����ˮ�Ҳ��μӷ�Ӧ����
��ͬѧ���е�ʵ���������������£���ÿ��ȡ�õ�ʯ��ʯ������ͬ��ϡ�������������Ҳ��ͬ��
| ʵ�� | ��һ�� | �ڶ��� | ������ |
| ȡ��ʯ��ʯ������/g | m | m | m |
| ȡ��ϡ���������/g | 30 | 60 | 90 |
| ��Ӧ����˵õ�������������Ѹ��/g | 6 | 2 | 1 |
�ٳ�ȡ����ΪW1g��ʯ��ʯ��Ʒ�������ձ��У�
�����ձ��м���40gϡ���ᣬ��ֽ��裬�����ݲ�����
�۳�ַ�Ӧ����ˡ�����Ƶù�������ΪW2g��
��______��
�����������㣺
��1����ͬѧʵ����̼��Ƶ���������______��
��2�������ͬѧʵ����еIJ�����Ԥ������______����ʵ�鲽���Ŀ����______��
��3���Ƚϼס���ͬѧʵ�鷽��������Ϊ______�����ͬѧ������ͬѧ���������Ϻã�������______��
��4�����ͬѧ����ϡ������������������������ݾ�ȷ��0.1%��
�⣺��ͬѧ���е�ʵ�飺��ͬѧҪ�ⶨʯ��ʯ��Ʒ��̼��Ƶ���������������ȷ��W2g�Ĺ��������в�����̼��ƣ���̼����������ᷴӦ�����ʿ�֪��������ǣ��ڹ����еμ�ϡ���ᣬ����ʣ�������û��̼���
�����������㣺
��1���ɱ����е����ݶԱȷ�����֪��ÿ30g����������4g��̼��Ʒ�Ӧ���ɴ˿�֪��m��ֵ��10g��������ʣ���1g���������ʣ����ԣ���ͬѧʵ����̼��Ƶ�����������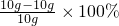 =90%��
=90%��
��2��������������֪�������ͬѧʵ����еIJ�����Ԥ�������ڹ����еμ�ϡ���ᣬû�����ݲ�������ʵ�鲽���Ŀ���ǣ�����ʣ�������û��̼��ƣ�ȷ��W2gȫ�������ʵ������Ӷ�ʹ�ⶨ��ʵ�����ݿɿ���
��3���Ƚϼס���ͬѧʵ�鷽������ͬѧ�ķ����Ϻã������ǣ���ͬѧ�IJ�����������õ�ҩƷҲ��Խ��٣�
��4����30 gϡ������HCl ������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 73
4g x
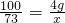 ��ã�x=2.97g
��ã�x=2.97g
ϡ���������ʵ���������Ϊ��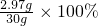 =9.9%��
=9.9%��
�ʴ�Ϊ�����ڹ����еμ�ϡ���ᣬ����ʣ�������û��̼��ƣ���1��90%����2���ڹ����еμ�ϡ���ᣬû�����ݲ����� ����ʣ�������û��̼��ƣ�ȷ��W2gȫ�������ʵ������Ӷ�ʹ�ⶨ��ʵ�����ݿɿ�����3����ͬѧ��������������õ�ҩƷҲ��Խ��٣���4����ͬѧ����ϡ�����������������Ϊ9.9%��
��������ͬѧ���е�ʵ�飺����̼���������ķ�Ӧ������ͬѧ��ʵ�鲽��ܣ�
�����������㣺
��1�����ݱ��е����ݶԱȷ���30g��������ȫ��Ӧʱ��Ӧ����̼��Ƶ�������ȷ����m��ֵ�����ʵ��������Ӷ������ͬѧʵ����̼��Ƶ�����������
��2������̼���������ķ�Ӧ��������ͬѧʵ����е�ʵ��������ʵ�鲽���Ŀ�ģ�
��3������ʵ��IJ��衢ҩƷ�������ȶԱȷ���������λͬѧ��ʵ�飻
��4����������������30g���ᷴӦ��̼��Ƶ����������30g���������ʵ��������������ϡ�������������������
�������������ڱ��������壬һЩ�������Ϣ�����������У�����Ĺؼ��ǣ�ͨ�����ݵĶԱ��ҳ����õ���Ϣ��������⣮
�����������㣺
��1���ɱ����е����ݶԱȷ�����֪��ÿ30g����������4g��̼��Ʒ�Ӧ���ɴ˿�֪��m��ֵ��10g��������ʣ���1g���������ʣ����ԣ���ͬѧʵ����̼��Ƶ�����������
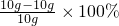 =90%��
=90%����2��������������֪�������ͬѧʵ����еIJ�����Ԥ�������ڹ����еμ�ϡ���ᣬû�����ݲ�������ʵ�鲽���Ŀ���ǣ�����ʣ�������û��̼��ƣ�ȷ��W2gȫ�������ʵ������Ӷ�ʹ�ⶨ��ʵ�����ݿɿ���
��3���Ƚϼס���ͬѧʵ�鷽������ͬѧ�ķ����Ϻã������ǣ���ͬѧ�IJ�����������õ�ҩƷҲ��Խ��٣�
��4����30 gϡ������HCl ������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 73
4g x
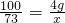 ��ã�x=2.97g
��ã�x=2.97gϡ���������ʵ���������Ϊ��
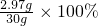 =9.9%��
=9.9%���ʴ�Ϊ�����ڹ����еμ�ϡ���ᣬ����ʣ�������û��̼��ƣ���1��90%����2���ڹ����еμ�ϡ���ᣬû�����ݲ����� ����ʣ�������û��̼��ƣ�ȷ��W2gȫ�������ʵ������Ӷ�ʹ�ⶨ��ʵ�����ݿɿ�����3����ͬѧ��������������õ�ҩƷҲ��Խ��٣���4����ͬѧ����ϡ�����������������Ϊ9.9%��
��������ͬѧ���е�ʵ�飺����̼���������ķ�Ӧ������ͬѧ��ʵ�鲽��ܣ�
�����������㣺
��1�����ݱ��е����ݶԱȷ���30g��������ȫ��Ӧʱ��Ӧ����̼��Ƶ�������ȷ����m��ֵ�����ʵ��������Ӷ������ͬѧʵ����̼��Ƶ�����������
��2������̼���������ķ�Ӧ��������ͬѧʵ����е�ʵ��������ʵ�鲽���Ŀ�ģ�
��3������ʵ��IJ��衢ҩƷ�������ȶԱȷ���������λͬѧ��ʵ�飻
��4����������������30g���ᷴӦ��̼��Ƶ����������30g���������ʵ��������������ϡ�������������������
�������������ڱ��������壬һЩ�������Ϣ�����������У�����Ĺؼ��ǣ�ͨ�����ݵĶԱ��ҳ����õ���Ϣ��������⣮

��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ