��Ŀ����
������δ���������Դ��ˮ��δ������֮Դ��
��1��д��A�����õ������Ļ�ѧ����ʽ��
��2������Ϊ�������Ԫ��ѭ������ͼ��ʾ���������Ԫ��ѭ�����̣��������Խ����ԴΣ���������봫ͳ�Ļ�ʯȼ����Ȼ����ԣ�
��3��ijЩ�����⻯����ˮ��Ӧ�����ɼ���������磺CaH2+2H2O Ca��OH��2+2H2����CaH2������Na2CO3��Һ֮�䷢����Ӧ���ܵĻ�ѧ����ʽ�ǣ�
���𰸡���������1�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ
��2��������Ϊȼ���������ŵ�ٷ��ȶ��ԭ�Ϲ�۲�����ˮ����Ⱦ
��3�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ
����⣺��1����ˮΪԭ����ȡ�����Ļ�ѧ����ʽΪ��2H2O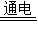 2H2��+O2�����ʴ�Ϊ��2H2O
2H2��+O2�����ʴ�Ϊ��2H2O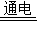 2H2��+O2����
2H2��+O2����
��2��������Ϊȼ���������ŵ�ٷ��ȶ��ԭ�Ϲ�۲�����ˮ����Ⱦ����ʯȼ��ȼ�ջ������Ⱦ��ʴ�Ϊ��B�����ٶԻ�������Ⱦ
��3��CaH2+2H2O�TCa��OH��2+2H2�������ɵ� Ca��OH��2��Na2CO3�ܷ�Ӧ����ѧ����ʽΪ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH����������ѧ����ʽ���ҷֱ���Ӽ��ɵó��𰸣��ʴ�Ϊ��CaH2+2H2O+Na2CO3=CaCO3��+2NaOH+2H2��
������������Ҫ�����˻�ѧ����ʽ����д��ȼ�ϵķ��������Ԫ����ԭ�ӵ���ϵ�ȷ�������ݣ�
��2��������Ϊȼ���������ŵ�ٷ��ȶ��ԭ�Ϲ�۲�����ˮ����Ⱦ
��3�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ
����⣺��1����ˮΪԭ����ȡ�����Ļ�ѧ����ʽΪ��2H2O
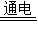 2H2��+O2�����ʴ�Ϊ��2H2O
2H2��+O2�����ʴ�Ϊ��2H2O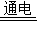 2H2��+O2����
2H2��+O2������2��������Ϊȼ���������ŵ�ٷ��ȶ��ԭ�Ϲ�۲�����ˮ����Ⱦ����ʯȼ��ȼ�ջ������Ⱦ��ʴ�Ϊ��B�����ٶԻ�������Ⱦ
��3��CaH2+2H2O�TCa��OH��2+2H2�������ɵ� Ca��OH��2��Na2CO3�ܷ�Ӧ����ѧ����ʽΪ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH����������ѧ����ʽ���ҷֱ���Ӽ��ɵó��𰸣��ʴ�Ϊ��CaH2+2H2O+Na2CO3=CaCO3��+2NaOH+2H2��
������������Ҫ�����˻�ѧ����ʽ����д��ȼ�ϵķ��������Ԫ����ԭ�ӵ���ϵ�ȷ�������ݣ�

��ϰ��ϵ�д�
�����Ŀ
 ȼ�ϵ�ȼ�����������ķ�չ�����������൱��Ҫ�����ã�
ȼ�ϵ�ȼ�����������ķ�չ�����������൱��Ҫ�����ã�