��Ŀ����
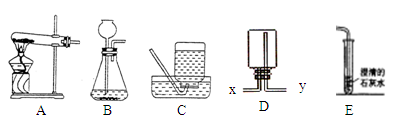
��10�֣�ijѧϰС����������װ�ý����������ȡ�о�����ش��������⡣
��1��д���б�ŵ��������� ��_____________ ��______________��
��2��ѡ�ø��������ȡ�����Ļ�ѧ����ʽ___________________________�� A��E���ӣ���ȡ������������ԭ�������______________��дһ�㼴�ɣ���
��3��ѡ��Bװ����ȡ�������ù���������Һ�Ͷ������̻�ϣ����ж������̵�������____________________��Ϊ�˿��Ƹ÷�Ӧ���ʣ��ɽ���Ţ���������___________��
��4������Fװ���ռ�������̼��������Ӧ��____��ͨ�루�a����b����������Fװ�ü����Ƶõ������Ƕ�����̼������Fװ���з�����Ӧ�Ļ�ѧ����ʽ______________��
��1���ٳ���©�� �ڼ���ƿ��2��2KMnO4 K2MnO4+ MnO2+O2�� �ղ������ݾ��ռ�����տ�ʼ����ƿ��δװ��ˮ����3�������� ��Һ©������ע������ (4) a CO2+Ca(OH)2===CaCO3��+H2O
K2MnO4+ MnO2+O2�� �ղ������ݾ��ռ�����տ�ʼ����ƿ��δװ��ˮ����3�������� ��Һ©������ע������ (4) a CO2+Ca(OH)2===CaCO3��+H2O
����

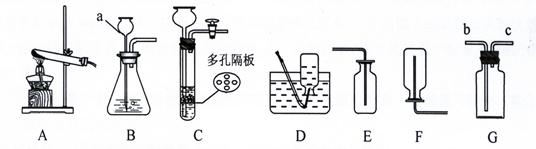
��8�֣����������ʵ��װ��ͼ�ش����⣺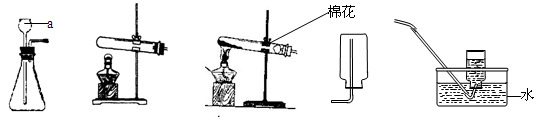
A B C D E
��1��д�����a���������ƣ�a ____________
��2���ø��������ȡ������Ӧѡ�õķ���װ�ú��ռ�װ����___ _____�����ţ�����������Ӧ�Ļ�ѧ����ʽΪ:__________ __ _________________��
��3��ʵ������ȡ������һ�㲽���У���װҩƷ �ڼ�������� �۹̶�װ�� �ܼ���
��Ϩ��ƾ��� �ްѵ��ܴ�ˮ�����ó� ���ռ����塣��ȷ��ʵ����������ǣ� ��
| A���٢ڢۢܢߢݢ� | B���ڢ٢ۢܢߢޢ� |
| C���٢ڢۢܢߢޢ� | D���ڢ٢ۢܢߢݢ� |
��ͼ��ʵ������ȡ���峣�õ�װ�ã���������ʻش��й�����
��1��д��������������ƣ��� ���� ��
��2�������Ѿ�ѧ��ʵ������ȡO2��H2��CO2��������Ļ�ѧ��Ӧԭ������д����ѧ��Ӧ����ʽ�� �� �� ��
ͨ���۲�Ƚϵó�����������ѧ��Ӧ�Ĺ�ͬ�� ������ţ���
A.��Ҫ���� B.��Ҫ���� C.û������μӷ�Ӧ D.���ɵ�����ֻ��һ��
��3��ʵ������ȡCO2�ķ���װ�ú��ռ�װ�÷ֱ��� ����װ�ô��ţ������������̼�ռ����ķ����� ��
��4������C��F��ϳ�װ������ȡ���壬Ӧ�������������е� �� ��
�ٷ�Ӧ���ǹ����Һ�� �ڷ�Ӧ����Ҫ����
���Ƶõ������ܶȱȿ���С ���Ƶõ����岻������ˮ
A���٢� B���٢ڢ� C���ڢ� D���٢ڢۢ�
��5��װ��C��B��ȣ��ŵ���
��6�����Ķ��±������Ϻش��������⣺
| ���� | ��ȡ�����ҩƷ | ��ȡ����ķ�Ӧ���� | ������������� |
| ���� | MnO2�����Ũ���� | ��Ҫ���� | ������ˮ���ܶȱȿ����� |
ʵ������ȡ����____________������Ի��ԣ�����װ��A��B��������___________��