��Ŀ����
��������װ��ͼ��գ�
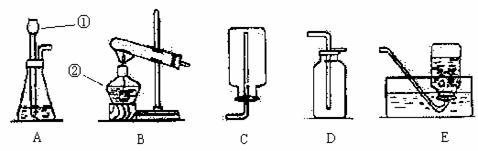
��1��д����Ţ١������������ƣ���________________����________________��
��2��д����װ��B��ȡ������һ����ѧ����ʽ______________________��Bװ�����Թܿ���������б��Ŀ����____________________________��
��3��ijͬѧ��Eװ�ü���һƿ������ʵ��ʱ��������������ԭ�������____________��
��4��ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼ʱ��Ӧѡ��ͼ��__________ʵ��װ�ã����������̼�����ķ�����____________________��
��5��ʵ������п����ϡ�����ڳ����·�Ӧ��ȡ��������ѡ�õķ���װ��Ϊ__________���ռ�װ��Ϊ___________��
��1���ٳ���©�����ھƾ���
��2��2KMnO4  K2MnO4 +MnO2 +O2������ֹ����ʱ����ˮ�������ȵ��Թܵײ���ʹ�Թ�ը�ѡ�
K2MnO4 +MnO2 +O2������ֹ����ʱ����ˮ�������ȵ��Թܵײ���ʹ�Թ�ը�ѡ�
��3��δ�ȵ������������ݾͿ�ʼ�ռ������������ɣ�
��4��A��D����ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��۲�ľ���Ƿ�Ϩ��
��5��A��C��E

��ϰ��ϵ�д�
�����Ŀ
 ��̼��____________________________________________��
��̼��____________________________________________��