��Ŀ����
��ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ��BaCl2+Na2CO3==BaCO3��+2NaCl��
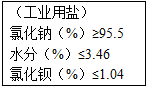
��
��1��100g�ù�ҵ���к��Ȼ�����������
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ
��1��100g�ù�ҵ���к��Ȼ�����������
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ
�𣺣�1��100g�ù�ҵ���к��Ȼ���������Ϊ1.04g��
��2���˹�ҵ�����Ȼ��Ƶ������������ϲ�Ʒ����ָ�꣮
��2���˹�ҵ�����Ȼ��Ƶ������������ϲ�Ʒ����ָ�꣮

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
��ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ��
BaCl2+Na2CO3=BaCO3��+2NaCl��
BaCl2+Na2CO3=BaCO3��+2NaCl��