��Ŀ����
����ʵ��װ��ͼ���ش��������⣺
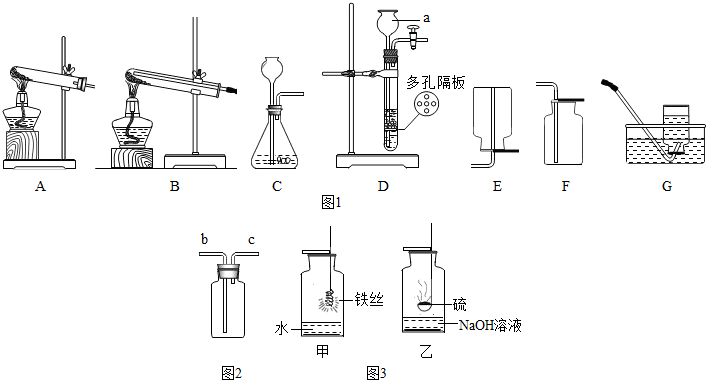
��ʵ�������ſ������ռ�������ѡ���������� ���� װ�ã��ñ�Żش𣩡�
��ʵ������KMnO4�������ķ�Ӧ����ʽΪ���� ������ ������ �� ������������KMnO4��������ʵ���У���������Թ����ѣ�ԭ���������������������������
���������������������������������������������������� ���������������������������������������������������� �������ʧ�����ԭ��
�ǿ�ѡ������ ���� װ�ó�ȥCO�л��е�CO2(�ñ�Żش�)����װ�������ʢ�������� ����������Һ��
��ʵ��������ˮ�����ƹ�������ʯ�Ҽ�����ȡ�������塣������ܶȱȿ���С��������ˮ����ȡ����ķ������ռ�װ�ÿ�ѡ���������� �������� �������������� ������������ ���ñ�Żش𣩡�
��Fװ�ÿ��ռ��ʹ�������������ˮ��װ���е������ų���ˮӦ��(��a��b)������ͨ�롣
�𰸣���1��D
��2��2KMnO4�TK2MnO4+MnO2+O2�� �г��Թܵ����й�����δ�����Թ��������͵���أ�ʹ�Թ��������ͷ���ը�ѣ����Թ��еĹ���ҩƷ����ʱ���Թܿ�û��������б�����Թܼ���ʱ��û�ȸ������Թܾ�������
��3��F NaOH��KOH
��4��A��C ��A��E
��5��a

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�





 ��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������
��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������