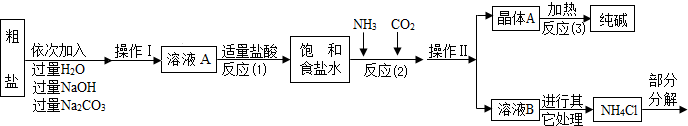
��Ŀ����
7����ͼ��ʾ����ijͬѧ��Ƶ���ȡ�����������CO2�����װ�ã��ش��������⣺

��1��ָ��ͼ�б������������ƣ�����
��2��ָ��ͼ��װ�õĴ���
��3��B��NaHCO3��Һ�������dz���CO2�л�������HCl���壬C��ŨH2SO4��������
��4����ͬѧ����������װ���еĴ�������ʵ��ʱ������D��CO2�Բ��ܼ��������ܵ�ԭ���Ǣ�

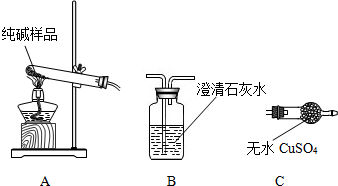
��1��ָ��ͼ�б������������ƣ�����
����©��
������
����ƿ
����2��ָ��ͼ��װ�õĴ���
����ƿ�е���û�������ײ�
����3��B��NaHCO3��Һ�������dz���CO2�л�������HCl���壬C��ŨH2SO4��������
����ˮ����
��B�е�NaHCO3��Һ���ܻ���ʯ��ˮ��������
������̼Ҳ�ܺ�ʯ��ˮ��Ӧ
����4����ͬѧ����������װ���еĴ�������ʵ��ʱ������D��CO2�Բ��ܼ��������ܵ�ԭ���Ǣ�
�����Բ���
����ҩƷ��������
��������������ȷÿ��װ�õ����ã�A�Ƿ���װ�ã�������ȡ������̼��B��C���Ǿ���װ�ã�B�������Ȼ������壬D������ˮ������D���ռ�װ�ã���������̼�е��Ȼ���Ҫ��̼��������Һ��������ʯ��ˮ������������Һ������Ѷ�����̼Ҳ�������ռ�������̼ʱ����Ҫ���뼯��ƿ�ײ���Ŀ����Ϊ�˸��õİѿ����ų����ռ��������Ķ�����̼��
����⣺��1�����ݳ��������������жϳ����������ƣ����� ����©�������� ����ƿ��
��2���ռ�������̼ʱ����Ҫ���뼯��ƿ�ײ�������Dװ�ô���
��3������Ũ���������ˮ�ԣ�����C��ŨH2SO4������������ˮ������B�л���ʯ��ˮʱ������HCl�����ͬʱ������̼Ҳ�������ˣ�
��4����ͬѧ����������װ���еĴ�������ʵ��ʱ������D��CO2�Բ��ܼ��������ܵ�ԭ����װ��©����ҩƷ����������
�𰸣���1������ ����©�������� ����ƿ��
��2������ƿ�е���û�������ײ���
��3�� ����ˮ������������̼Ҳ�ܺ�ʯ��ˮ��Ӧ��
��4���������Բ��ã���ҩƷ��������
��2���ռ�������̼ʱ����Ҫ���뼯��ƿ�ײ�������Dװ�ô���
��3������Ũ���������ˮ�ԣ�����C��ŨH2SO4������������ˮ������B�л���ʯ��ˮʱ������HCl�����ͬʱ������̼Ҳ�������ˣ�
��4����ͬѧ����������װ���еĴ�������ʵ��ʱ������D��CO2�Բ��ܼ��������ܵ�ԭ����װ��©����ҩƷ����������
�𰸣���1������ ����©�������� ����ƿ��
��2������ƿ�е���û�������ײ���
��3�� ����ˮ������������̼Ҳ�ܺ�ʯ��ˮ��Ӧ��
��4���������Բ��ã���ҩƷ��������
�����������ۺϿ�����ʵ������ȡ������̼���й�֪ʶ����������չ�������ͬѧ�ǵ���ʶˮƽ��

��ϰ��ϵ�д�
�����Ŀ
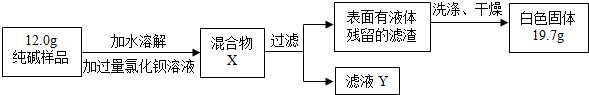
 ijͬѧҪ���Ȼ��ƹ�������������������Ϊ16%��ʳ����Һ��
ijͬѧҪ���Ȼ��ƹ�������������������Ϊ16%��ʳ����Һ��