��Ŀ����
��2010?��ҵ��һģ����֪ij������ĩ�г�����Al�����һ������Fe��Cu��Ϊ�ⶨ����Al������������ij��ѧ��ȤС���ͬѧչ�������µ�ʵ��̽�����������ߣ�Al������������Һ��Ӧ��������ˮ��ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2A1+2H2O+2NaOH�T2NaAlO2+3H2������Fe��Cu��������������Һ��Ӧ��ȡ20g�ý�����ĩ����100g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ��
| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ʣ����������/g | 16.5 | n | 9.5 | �� |
��2���ý�����ĩ��Al����������Ϊ______%��
��3����ʽ���㣺��������������Һ��������������Ϊ���٣�����ȷ��0.1%����

���𰸡���������1�������������ݿ�֪����һ�η�Ӧ��ȥ����3.5g���壬�ڶ��Σ�ҲӦ��Ӧ������3.5g���ҳ����ɣ�ÿ�μ��ٹ���3.5g�������ϱ���n��ֵΪ13��
��2����ͼ�п�֪��ʣ������Ϊ����Ϊ6g������������Ϊ20g-6g����ý�����ĩ��Al������������
��3�����û�ѧ����ʽ�Ľ��м��㣺������������Һ��������������Ϊx����ȷ��д��ѧ����ʽ��������֪����δ֪�����б���ʽ������������������Һ����������������
����⣺��1�������������ݿ�֪����һ�η�Ӧ��ȥ3.5g���壬�ڶ��Σ�ҲӦ��Ӧ��3.5g�������ϱ���n��ֵΪ13��
��2����ͼ�п�֪���ý�����ĩ��Al����������Ϊ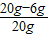 ×100%=70%��
×100%=70%��
��3��������������Һ��������������Ϊx
2Al+2H2O+2NaOH=2NaAlO2+3H2��
54 80
20g-6g 80xg
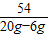 =
=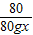 ���x=25.9%
���x=25.9%
������������Һ��������������Ϊ25.9%
�ʴ�Ϊ����1��13����2��70����3��25.9%
������������Ҫ�����˽����Ļ�ѧ���ʺͽ������˳����Ӧ�ã������й��������������ļ�����ݻ�ѧ����ʽ�ļ��㣮
��2����ͼ�п�֪��ʣ������Ϊ����Ϊ6g������������Ϊ20g-6g����ý�����ĩ��Al������������
��3�����û�ѧ����ʽ�Ľ��м��㣺������������Һ��������������Ϊx����ȷ��д��ѧ����ʽ��������֪����δ֪�����б���ʽ������������������Һ����������������
����⣺��1�������������ݿ�֪����һ�η�Ӧ��ȥ3.5g���壬�ڶ��Σ�ҲӦ��Ӧ��3.5g�������ϱ���n��ֵΪ13��
��2����ͼ�п�֪���ý�����ĩ��Al����������Ϊ
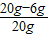 ×100%=70%��
×100%=70%����3��������������Һ��������������Ϊx
2Al+2H2O+2NaOH=2NaAlO2+3H2��
54 80
20g-6g 80xg
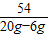 =
=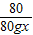 ���x=25.9%
���x=25.9%������������Һ��������������Ϊ25.9%
�ʴ�Ϊ����1��13����2��70����3��25.9%
������������Ҫ�����˽����Ļ�ѧ���ʺͽ������˳����Ӧ�ã������й��������������ļ�����ݻ�ѧ����ʽ�ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
��2010?��ҵ��ģ�⣩ij��ȤС��Χ���š������ڿ����еı�ը���ޡ�����̽������¼��������������±���ʾ��
��1������˵����ȷ���ǣ�______
A����ȼ�����Ϳ����Ļ�����岻һ���ᷢ����ը
B����ȼ����ǰ��һ��Ҫ���������Ĵ���
C����ȼ���������������������30%�Ļ������ᷢ����ը
D��ֻ�о��Դ�������������ȼ��Ų��ᷢ����ը
��2����֪�����������������������������ˮʱ�Ļ�ѧ������֮�Ƚ��л��ʱ����ը����ң�
������������������������Ϊ20%�����Ϊ����������200L�����л������������ʱ��ը����ң�
| H2���������%�� | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| �������������%�� | 95 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | 10 |
| ��ȼʱ���� | ��ȼ ���� | ����ը | ǿ��ը | ǿ��ը | ǿ��ը | ǿ��ը | ǿ��ը | ����ը | ���� ȼ�� | ���� ȼ�� |
A����ȼ�����Ϳ����Ļ�����岻һ���ᷢ����ը
B����ȼ����ǰ��һ��Ҫ���������Ĵ���
C����ȼ���������������������30%�Ļ������ᷢ����ը
D��ֻ�о��Դ�������������ȼ��Ų��ᷢ����ը
��2����֪�����������������������������ˮʱ�Ļ�ѧ������֮�Ƚ��л��ʱ����ը����ң�
������������������������Ϊ20%�����Ϊ����������200L�����л������������ʱ��ը����ң�
��2010?��ҵ��ģ�⣩��֪�����������ܺ�ˮ��Ӧ������Ӧ���ᣬ��������ᡢ����һ������ʹ���ָʾ����ɫ�������������Ʒ����кͷ�Ӧ��������ͼ��������������ʵ�飬���ŷ�Ӧ�Ľ��У����ι۲쵽�±��м�¼��ʵ��������ѡ������·���A��B��C�Ƚ��Ͳ�����Щ�����ԭ���ж��Ҫ��ʱ��һ��ѡ������
A�������ĺ���ȼ�պ���ƿ�ڿ����е�������ȫ����
B��������������ˮ��Ӧ��������
C����ȴ����ƿ�������ѹǿ��С
D������ƿ�������������͵�������ڸտ�ʼȼ��ʱ���ĵ����������
E������ȼ�շų���������
F������ȼ�����ɰ�ɫ��������������
H�������������ĺ���Լռ������������֮һ
I��������������һ��ʹ��ɫʯ����Һ���ɫ��

| ���� | ԭ�� | ||
| ʵ�� �տ� ʼʱ | 1 | ����ƿ���д������� | |
| 2 | ����ƿ���� | ||
| 3 | �����ձ��еĵ��ܿ�������ð�� | ||
| һ�� ʱ�� �Ժ� | 4 | �ձ���ˮ��Һ�����ؼ���ƿ�� | |
| 5 | �����ؼ���ƿ�е���Һ�[��ɫ | ||
| 6 | �����뼯��ƿ��Һ������Լռ����ƿ�ݻ������֮һ | ||
B��������������ˮ��Ӧ��������
C����ȴ����ƿ�������ѹǿ��С
D������ƿ�������������͵�������ڸտ�ʼȼ��ʱ���ĵ����������
E������ȼ�շų���������
F������ȼ�����ɰ�ɫ��������������
H�������������ĺ���Լռ������������֮һ
I��������������һ��ʹ��ɫʯ����Һ���ɫ��
