��Ŀ����
��2013?�γ�ģ�⣩����������һ����Ҫ�Ļ���ԭ�ϣ�
�������������ƿ���ʯ��ʯ��ˮ�����Na2CO3��Ϊԭ����ȡ���������£�
���û�ѧ����ʽ��ʾ��ɫ������봿����Һ�����з����Ļ�ѧ��Ӧ
����Һ�д���������Na2CO3��������
��Ϊ�ⶨij���ֱ��ʵ��������ƹ������������Ƶ������������������װ�ý���ʵ�飨����ÿ��ʵ�鶼��ȫ���У�Ũ���������ˮ�ԣ���

��Ҫȷ������Ʒ���������Ƶ���������������Ҫ��������
A��������Ʒ������ B��Ũ����ʵ��ǰ��������C��Ũ����������Һʵ��ǰ������
D������ϡ�������� E������D���������ƹ���ʵ��ǰ������ F��ϡ������������
��Dװ�õ�������
�������������ƿ���ʯ��ʯ��ˮ�����Na2CO3��Ϊԭ����ȡ���������£�

���û�ѧ����ʽ��ʾ��ɫ������봿����Һ�����з����Ļ�ѧ��Ӧ
CaO+H2O=Ca��OH��2 ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH
CaO+H2O=Ca��OH��2 ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH
������Һ�д���������Na2CO3��������
Ca��OH��2
Ca��OH��2
�Լ�����ȥ�������ʣ���Ϊ�ⶨij���ֱ��ʵ��������ƹ������������Ƶ������������������װ�ý���ʵ�飨����ÿ��ʵ�鶼��ȫ���У�Ũ���������ˮ�ԣ���

��Ҫȷ������Ʒ���������Ƶ���������������Ҫ��������
A��C
A��C
��A��������Ʒ������ B��Ũ����ʵ��ǰ��������C��Ũ����������Һʵ��ǰ������
D������ϡ�������� E������D���������ƹ���ʵ��ǰ������ F��ϡ������������
��Dװ�õ�������
���տ����е�ˮ�Ͷ�����̼
���տ����е�ˮ�Ͷ�����̼
��û��Dװ�ö������������������IJⶨ���Ӱ����ƫС
ƫС
���ƫ��ƫС�����䡱�����������٢�ʯ��ʯ���·ֽ������˰�ɫ���������ƣ��������Ƽ��봿����Һ�������ƺ�ˮ��Ӧ�����������ƣ��������ƺ�̼���Ʒ�Ӧ������̼��Ƴ������������ƣ�д����Ӧ�ķ���ʽ��
�����������ƺ�̼���Ƶķ�Ӧ���з�����
�ڢ�����Ʒ���������Ƶ������������㹫ʽ�������Ĺ��̷�����Ҫ�����ݣ�
����ʵ���ԭ�����������Ƶ����ʽ��з�����
�����������ƺ�̼���Ƶķ�Ӧ���з�����
�ڢ�����Ʒ���������Ƶ������������㹫ʽ�������Ĺ��̷�����Ҫ�����ݣ�
����ʵ���ԭ�����������Ƶ����ʽ��з�����
����⣺�٢��������֪��ʯ��ʯ���·ֽ����˰�ɫ���������ƣ��������Ƽ��봿����Һ�������ƺ�ˮ��Ӧ�����������ƣ��������ƺ�̼���Ʒ�Ӧ������̼��Ƴ������������ƣ����ԣ������ķ�Ӧ����ʽ�ǣ�CaO+H2O=Ca��OH��2 ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH��
����Һ�д���������Na2CO3��������Ca��OH��2 �Լ�����ȥ�������ʣ�
�ڢ�����Ʒ���������Ƶ������������㹫ʽ��֪����Ҫ�������ǣ�������Ʒ������������ʵ��Ĺ��̣���Ũ����������Һʵ��ǰ�������ı仯�������̼���Ƶ���������������������Ƶ����������Ϳ��������Ʒ���������Ƶ��������������ԣ�����Ҫ��������A��C��
��Ϊ�˷�ֹ������ˮ�Ͷ�����̼Ӱ��ʵ��Ľ���������������ƹ����������տ����е�ˮ�Ͷ�����̼��������ʵ����̿�֪�����û��Dװ�ã��������������������IJⶨ���Ӱ����ƫС��
�ʴ�Ϊ���٢�CaO+H2O=Ca��OH��2 ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����Ca��OH��2���ڢ�A��C�������տ����е�ˮ�Ͷ�����̼��ƫС��
����Һ�д���������Na2CO3��������Ca��OH��2 �Լ�����ȥ�������ʣ�
�ڢ�����Ʒ���������Ƶ������������㹫ʽ��֪����Ҫ�������ǣ�������Ʒ������������ʵ��Ĺ��̣���Ũ����������Һʵ��ǰ�������ı仯�������̼���Ƶ���������������������Ƶ����������Ϳ��������Ʒ���������Ƶ��������������ԣ�����Ҫ��������A��C��
��Ϊ�˷�ֹ������ˮ�Ͷ�����̼Ӱ��ʵ��Ľ���������������ƹ����������տ����е�ˮ�Ͷ�����̼��������ʵ����̿�֪�����û��Dװ�ã��������������������IJⶨ���Ӱ����ƫС��
�ʴ�Ϊ���٢�CaO+H2O=Ca��OH��2 ��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����Ca��OH��2���ڢ�A��C�������տ����е�ˮ�Ͷ�����̼��ƫС��
����������Ƚ�ȫ��Ŀ������������Ƶ���ȡ�����ʣ����������ڿ������׳��⣬Ҳ�����տ����еĶ�����̼������̼���ƶ����ʣ������ڼ����Ƿ����ʱ��ֻ�������̼������ɣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
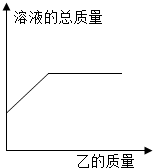 ��2013?�γ�ģ�⣩��һ�������ļ���Һ����������������ʵ���������Һ��������������ҵ�������ϵ��������ͼ���߱�ʾ���� ��������
��2013?�γ�ģ�⣩��һ�������ļ���Һ����������������ʵ���������Һ��������������ҵ�������ϵ��������ͼ���߱�ʾ���� �������� ��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�
��2013?�γ�ģ�⣩ʵ���ҳ��ñ��������������Ȼ����Һ��Ӧ��ȡ�����ĵ�������Ӧ�Ļ�ѧ����ʽΪ��NaNO2+NH4Cl=NaCl+N2+2H2O���˷�Ӧ�Ƿ��ȷ�Ӧ����ʵ��װ����ͼ��ʾ���Իش�