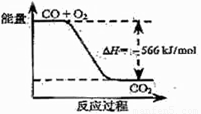
摘要:3.已知:①C(S)+ 1/2 O2 △H = -110.5kJ/mol .
网址:http://m.1010jiajiao.com/timu_id_443573[举报]
已知:①C(s)+1/2O2(g)══CO(g) ΔΗ1="-110.35" kJ·mol-1
②CO(g)+1/2O2(g)══CO2(g) ΔΗ2="?-282.57" kJ·mol-1
③C(s)+O2(g)══CO2(g) ΔΗ3 则ΔΗ3?等于( )
②CO(g)+1/2O2(g)══CO2(g) ΔΗ2="?-282.57" kJ·mol-1
③C(s)+O2(g)══CO2(g) ΔΗ3 则ΔΗ3?等于( )
| A.+172.22 kJ·mol-1 |
| B.-172.22 kJ·mol-1 |
| C.+392.92 kJ·mol-1 |
| D.-392.92 kJ·mol-1 |
已知:①C(s)+1/2O2(g)══CO(g) ΔΗ1="-110.35" kJ·mol-1
②CO(g)+1/2O2(g)══CO2(g) ΔΗ2="-282.57" kJ·mol-1
③C(s)+O2(g)══CO2(g) ΔΗ3 则ΔΗ3等于
- A.+172.22 kJ·mol-1
- B.-172.22 kJ·mol-1
- C.+392.92 kJ·mol-1
- D.-392.92 kJ·mol-1
已知:2CO(g)+O2(g)=2CO2(g) ΔH= -- 566 kJ/mol
Na2O2(s)+CO2(g)=Na2CO3(s)+ 1/2O2(g) ΔH= --226 kJ/mol
根据以上热化学方程式判断,下列说法正确的是( )
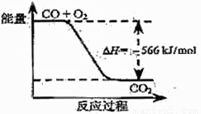
A.CO的燃烧热为283 kJ
B.右图可表示由CO生成CO2的反应过程和能量关系
C.2Na2O2(s)+2CO2(s)=2Na2CO3(s)+O2(g) ΔH< --452 kJ/mol
D.CO(g)与Na2O2(s)反应放出509 kJ热量时,电子转移数为2×6.02×1023
查看习题详情和答案>>