摘要:19.已知热化学方程式: ①H2(g)+O2(g)=H2O(g); △H=-241.8kJ·mol-1 ②2H2(g)+O2(g)=2H2O(g); △H=-483.6kJ·mol-1 ③H2(g)+ O2(g)=H2O(1); △H=-285.8kJ·mol-1 k+s-5#u ④2H2(g)+O2(g)=2H2O(1); △H=-571.6kJ·mol-1 则氢气的燃烧热为 ( ) A.241.8kJ·mol-1 B.483.6kJ·mol-1 C.285.8kJ·mol-1 D.571.6kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_56699[举报]
已知热化学方程式:
①H2(g)+![]() O2(g)
O2(g)![]() H2O(g) ΔH=-241.8 kJ·mol-1
H2O(g) ΔH=-241.8 kJ·mol-1
②2H2(g)+O2(g)![]() 2H2O(g) ΔH=-483.6 kJ·mol-1
2H2O(g) ΔH=-483.6 kJ·mol-1
③H2(g)+ ![]() O2(g)
O2(g)![]() H2O(l) ΔH=-285.8 kJ·mol-1
H2O(l) ΔH=-285.8 kJ·mol-1
④2H2(g)+O2(g)![]() 2H2O(l) ΔH=-571.6 kJ·mol-1
2H2O(l) ΔH=-571.6 kJ·mol-1
则氢气的燃烧热为( )
A.241.8 kJ·mol-1 B.483.6 kJ·mol-1 C.285.8 kJ·mol-1 D.571.6 kJ·mol-1
查看习题详情和答案>>已知热化学方程式:
① C2H2(g) + O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ 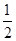 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) +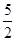 O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+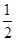 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
① C2H2(g) +
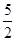 O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+
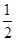 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) +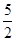 O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
① C2H2(g) +
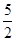 O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) + O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |