摘要:11.已知下列热化学方程式: (1)CH3COOH(l)+2O2(g) === 2CO2(g)+2H2O(l) △H1= -870.3 kJ·mol-1 +O2(g) === CO2(g) △H2= -393.5 kJ·mol-1 (3)H2(g)+O2(g) === H2O(l) △H3= -285.8 kJ·mol-1 则反应2C(s)+2H2(g)+O2(g)===CH3COOH(l) 的△H为( )kJ·mol-1 A.+488.3 B.-244.15 C.-488.3 D. +244.15
网址:http://m.1010jiajiao.com/timu3_id_419392[举报]
已知下列热化学方程式:
①CH3COOH(l)+2O2(g)===2CO2(g)+2H2O(l) ΔH1=-870.3 kJ/mol
②C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol
③H2(g)+ O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
则反应④2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变为
| A.488.3 kJ/mol | B.-224.15 kJ/mol |
| C.-488.3 kJ/mol | D.244.15 kJ/mol |
已知下列热化学方程式:
①CH3COOH(l)+2O2(g)===2CO2(g)+2H2O(l) ΔH1=-870.3 kJ/mol
②C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol
③H2(g)+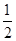 O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
则反应④2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变为
A.488.3 kJ/mol B.-224.15 kJ/mol
C.-488.3 kJ/mol D.244.15 kJ/mol
查看习题详情和答案>>
已知下列热化学方程式:
①CH3COOH(l)+2O2(g)===2CO2(g)+2H2O(l) ΔH1=-870.3 kJ/mol
②C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol
③H2(g)+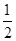 O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
则反应④2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变为
①CH3COOH(l)+2O2(g)===2CO2(g)+2H2O(l) ΔH1=-870.3 kJ/mol
②C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol
③H2(g)+
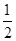 O2(g)===H2O(l) ΔH3=-285.8 kJ/mol
O2(g)===H2O(l) ΔH3=-285.8 kJ/mol则反应④2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变为
| A.488.3 kJ/mol | B.-224.15 kJ/mol |
| C.-488.3 kJ/mol | D.244.15 kJ/mol |