网址:http://m.1010jiajiao.com/timu3_id_410153[举报]
25℃,101kPa时,强酸与强碱的稀溶液发生中和反应生成1mol水时所放出的热量约为57.3 kJ/mol;辛烷的燃烧热为5518 kJ/mol。下列热化学方程式书写正确的是( )
A.CH3COOH(aq)+NaOH(aq)="=="
CH3COONa(aq)+H2O(l) 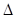 H=
H=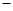 57.3kJ/mol
57.3kJ/mol
B.KOH(aq)+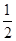 H
H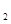 SO4(aq)=
SO4(aq)= 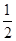 K2SO4(aq)+H2O(l)
K2SO4(aq)+H2O(l)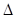 H=
H=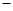 57.3kJ/mol
57.3kJ/mol
C.C8H18(l)+
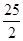 O2 (g)=8CO2 (g)+ 9H2O(g)
O2 (g)=8CO2 (g)+ 9H2O(g)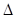 H=
H=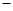 5518 kJ/mol
5518 kJ/mol
D.2C8H18(g)+25O2
(g)=16CO2 (g)+18H2O(1)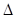 H=
H=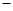 11036 kJ/mol
11036 kJ/mol
查看习题详情和答案>>
kJ/mol。下列热化学方程式书写正确的是
B.KOH(aq)+1/2H2SO4(aq)=1/2K2SO4(aq)+H2O(l) △H=57.3kJ/mol
C.C8H18(l)+25/2O2(g)=8CO2(g)+9H2O △H=5518 kJ/mol
D.2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1) △H=5518 kJ/mol
25℃,,101kPa时,强酸与强碱的稀溶液发生中和反应的中和热为57.3kJ·mol-1,辛烷的燃烧热为5 518 kJ·mol-1。下列热化学方程式书写正确的是( )
A.2H+(aq) + SO42-(aq) + Ba2+(aq) + 2OH-(aq)===BaSO4(s) + 2H2O(l);△H = -57.3kJ·mol-1
B.KOH(aq) + ![]() H2SO4(aq)===
H2SO4(aq)=== ![]() K2SO4(aq) + H2O(l);△H = -57.3kJ·mol-1
K2SO4(aq) + H2O(l);△H = -57.3kJ·mol-1
C.C3H8(l) + ![]() O2(g)===8CO2(g) + 9H2O(g);△H= -5 518 kJ·mol-1
O2(g)===8CO2(g) + 9H2O(g);△H= -5 518 kJ·mol-1
D.2C3H8(l) + 25O2(g)===16CO2(g) + 18H2O(l);△H= -5 518 kJ·mol-1
查看习题详情和答案>>②C8H18(l)+ 25/2O2(g)= 8CO2(g)+ 9H2O(l); △H = -5518kJ/mol
③H+(aq)+ OH-(aq)= H2O(l); △H = -57.3kJ/mol
④1/2H2SO4(aq) + NaOH(aq) =1/2Na2SO4(aq) + H2O(l); △H = +57.3kJ/mol
A.①③
B.②③
C.②④
D.②
已知101KPa时的辛烷的燃烧热为-5518kJ/mol,强酸与强碱在稀溶液中发生反应时的中和热为-57.3kJ/mol,则下列热化学方程式书写正确的是 ( )
①C8H18(l)+ 25/2O2(g)= 8CO2(g)+ 9H2O(l); △H = +5518kJ/mol
②C8H18(l)+ 25/2O2(g)= 8CO2(g)+ 9H2O(l); △H = -5518kJ/mol
③H+(aq)+ OH―(aq)= H2O(l); △H = -57.3kJ/mol
④1/2H2SO4(aq) + NaOH(aq) =1/2Na2SO4(aq) + H2O(l); △H = +57.3kJ/mol
A.①③ B.②③ C.②④ D.②
查看习题详情和答案>>