网址:http://m.1010jiajiao.com/timu3_id_385810[举报]
N2(g)+2O2(g)![]() N2O4(g) ΔH=+8.7 kJ·mol-1
N2O4(g) ΔH=+8.7 kJ·mol-1
N2H4(g)+O2(g) ![]() N2(g)+2H2O(g) ΔH=-534.0 kJ·mol-1
N2(g)+2H2O(g) ΔH=-534.0 kJ·mol-1
下列表示肼跟N2O4反应的热化学方程式,正确的是 …( )
A.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ΔH=-542.7 kJ·mol-1
3N2(g)+4H2O(g) ΔH=-542.7 kJ·mol-1
B.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ΔH=-1 059.3 kJ·mol-1
3N2(g)+4H2O(g) ΔH=-1 059.3 kJ·mol-1
C.N2H4(g)+![]() N2O4(g)
N2O4(g) ![]()
![]() N2(g)+2H2O(g) ΔH=-1 076.7 kJ·mol-1
N2(g)+2H2O(g) ΔH=-1 076.7 kJ·mol-1
D.2N2H4(g)+N2O4(g) ![]() 3N2(g)+4H2O(g) ΔH=-1 076.7 kJ·mol-1
3N2(g)+4H2O(g) ΔH=-1 076.7 kJ·mol-1
N2(g)+2O2(g) ══N2O4(g);ΔH=+8.7 kJ·mol -1
N2H4(g)+O2(g) ══N2(g)+2H2O(g);ΔH=-534.0 kJ·mol -1???
下列表示肼跟N2O4反应的热化学方程式,正确的是
A.2N2H4(g)+N2O4(g) ══3N2(g)+4H2O(g);ΔH=-542.7 kJ·mol -1??
B.2N2H4(g)+N2O4(g) ══3N2(g)+4H2O(g);ΔH=-1059.3 kJ·mol -1??
C.N2H4(g)+ ![]() N2O4(g) ══
N2O4(g) ══![]() N2(g)+2H2O(g);ΔH=-1076.7 kJ·mol -1??
N2(g)+2H2O(g);ΔH=-1076.7 kJ·mol -1??
D.2N2H4(g)+N2O4(g) ══3N2(g)+4H2O(g);ΔH=-1076.7 kJ·mol -1?
查看习题详情和答案>>N2(g)+2O2(g)====N2O4(g);ΔH=+8.7 kJ·mol-1
N2H4(g)+O2(g)====N2(g)+2H2O(g);ΔH=-534.0 kJ·mol-1
下列表示肼跟N2O4反应的热化学方程式,正确的是( )
A.2N2H4(g)+N2O4(g)====3N2(g)+4H2O(g);ΔH=-542.7 kJ·mol-1
B.2N2H4(g)+N2O4(g)====3N2(g)+4H2O(g);ΔH=-1 059.3 kJ·mol-1
C.N2H4(g)+![]() N2O4(g)====
N2O4(g)====![]() N2(g)+2H2O(g);ΔH=-1 076.7 kJ·mol-1
N2(g)+2H2O(g);ΔH=-1 076.7 kJ·mol-1
D.2N2H4(g)+N2O4(g)====3N2(g)+4H2O(g);ΔH=-1 076.7 kJ·mol-1
查看习题详情和答案>>肼(N2H4)是火箭发动机的一种燃料,反应时N2O4为氧化剂,生成N2和水蒸气。已知:N2(g)+2O2(g)=N2O4(g) ΔH="+8.7" kJ·mol-1
N2H4(g)+O2(g)=N2(g)+2H2O(g) ΔH="-534.0" kJ·mol-1
下列表示肼跟N2O4反应的热化学方程式,正确的是 ( )
| A.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH="-542.7" kJ·mol-1 |
| B.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH="-1059.3" kJ·mol-1 |
C.N2H4(g)+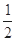 N2O4(g)= N2O4(g)=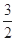 N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1 N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1 |
| D.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH="-1076.7" kJ·mol-1 |
肼(N2H4)是火箭发动机的一种燃料,反应时N2O4为氧化剂,生成N2和水蒸气。已知:
N2(g)+2O2(g)====N2O4(g) ΔH="+8.7" kJ·mol-1
N2H4(g)+O2(g) ====N2(g)+2H2O(g) ΔH="-534.0" kJ·mol-1
下列表示肼跟N2O4反应的热化学方程式,正确的是
| A.2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g) ΔH="-542.7" kJ·mol-1 |
| B.2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g)ΔH="-1059.3" kJ·mol-1 |
C.N2H4(g)+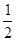 N2O4(g) ==== N2O4(g) ====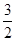 N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1 N2(g)+2H2O(g)ΔH="-1076.7" kJ·mol-1 |
| D.2N2H4(g)+N2O4(g) ====3N2(g)+4H2O(g)ΔH="-1076.7" kJ·mol-1 |