网址:http://m.1010jiajiao.com/timu3_id_36956[举报]
 有机物A常用于食品行业.已知9.0g A在足量O2中充分燃烧,将生成的混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4g和13.2g,经检验剩余气体为O2.
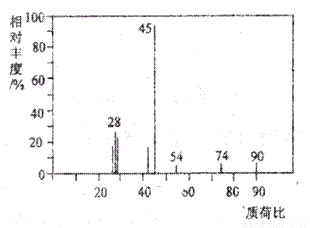
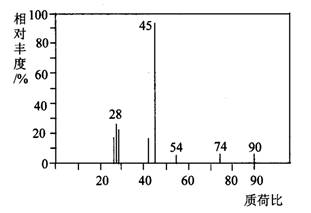
有机物A常用于食品行业.已知9.0g A在足量O2中充分燃烧,将生成的混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4g和13.2g,经检验剩余气体为O2.(1)A分子的质谱图如图所示,从图中可知其相对分子质量是90,则A的分子式是
(2)A能与NaHCO3溶液发生反应,A一定含有的官能团名称是
(3)A分子的核磁共振氢谱有4个峰,峰面积之比是1:1:1:3,则A的结构简式是
(4)0.1mol A与足量Na反应,在标准状况下产生H2的体积是
(5)A在一定条件下可聚合得到一种聚酯,用于制造手术缝合线,其反应的化学方程式是
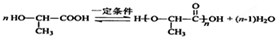
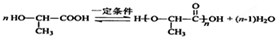
(10分)有机物A常用于食品行业。已知9.0gA在足量O2中充分燃烧,混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4g和13.2g,经检验剩余气体为O2。
(1)A分子的质谱图如下图所示,从图中可知其相对分子质量是90,则A的分子式是______________。

(2)A能与NaHCO3溶液发生反应,A一定含有的官能团名称是_____________。
(3)A分子的核磁共振氢谱有4个锋,峰面积之比是1:1:1:3,则A的结构简式是_______________。
(4)0.1mol A与足量Na反应,在标准状况下产生H2的体积是__________________L。
(5)A在一定条件下可聚合得到一种聚酯,用于制造手术缝合线,其反应的化学方程式是___________________。
查看习题详情和答案>>
(10分)有机物A常用于食品行业。已知9.0 g A在足量O2中充分燃烧,混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4 g和13.2 g,经检验剩余气体为O2。
(1)A分子的质谱图如下图所示,从图中可知其相对分子质量是90,则A的分子式是______________________。
(2)A能与NaHCO3溶液发生反应,A一定含有的官能团名称是__________________。
(3)A分子的核磁共振氢谱有4个峰,峰面积之比是1:1:1:3,则A的结构简式是_________________________________。
(4)0.1 mol A与足量Na反应,在标准状况下产生H2的体积是__________L。
(5)A在一定条件下可聚合得到一种聚酯,用于制造手术缝合线,其反应的化学方程式是___________________________________________________________________。
查看习题详情和答案>>
(10分)有机物A常用于食品行业。已知9.0 g A在足量O2中充分燃烧,将生成的混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4 g和13.2 g,经检验剩余气体为O2。
(1)A分子的质谱图如下图所示,从图中可知其相对
分子质量是90,则A的分子式是______________________。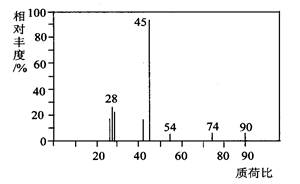
(2)A能与NaHCO3溶液发生反应,A一定含有的官能团名称是______________。
(3)A分子的核磁共振氢谱有4个峰,峰面积之比是1:1:1:3,则A的结构简式是_________________________________。
(4)0.1 mol A与足量Na反应,在标准状况下产生H2的体积是__________L。
(5)A在一定条件下可聚合得到一种聚酯,用于制造手术缝合线,其反应的化学方程式是___________________________。
(10分)有机物A常用于食品行业。已知9.0 g A在足量O2中充分燃烧,混合气体依次通过足量的浓硫酸和碱石灰,分别增重5.4 g和13.2 g,经检验剩余气体为O2。
(1)A分子的质谱图如下图所示,从图中可知其相对分子质量是90,则A的分子式是_______________。
(2)A能与NaHCO3溶液发生反应,A一定含有的官能团名称是__________________。
(3)A分子的核磁共振氢谱有4个峰,峰面积之比是1:1:1:3,则A 的结构简式是_________________________________。
(4)0.1 mol A与足量Na反应,在标准状况下产生H2的体积是__________L。
(5)A在一定条件下可聚合得到一种聚酯,用于制造手术缝合线,其反应的化学方程式是________________________________________。