摘要:9. 1g H2燃烧生成液态水放出142.9kJ的热量.下列热化学方程式正确的是 A.2H2(g)+ O 2(g) = 2H2O(1) H = -142.9kJ·molˉl B.2H2(g)+ O 2(g) = 2H2O(1) H = -571.6kJ·molˉl C.2H2+O2 = 2H2O H = -571.6lkJ·molˉl D.H2(g)+O 2(g) = H2O(1) H = +285.8kJ·molˉl
网址:http://m.1010jiajiao.com/timu3_id_310478[举报]
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是( )
A.2H2(g)+ O 2(g) =" " 2H2O(1)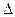 H =" " —142.9kJ·molˉl H =" " —142.9kJ·molˉl |
B.2H2(g)+ O 2(g) =" " 2H2O(1)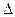 H =" " —571.6kJ·molˉl H =" " —571.6kJ·molˉl |
C.2H2+O2 = 2H2O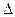 H = —571.6lkJ·molˉl H = —571.6lkJ·molˉl |
D.H2(g)+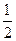 O 2(g) = H2O(1) O 2(g) = H2O(1)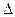 H = +285.8kJ·molˉl H = +285.8kJ·molˉl |
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是
A.2H2(g)+ O 2(g) = 2H2O(1)
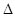 H = —142.9kJ·molˉl
H = —142.9kJ·molˉl
B.2H2(g)+ O 2(g) = 2H2O(1)
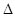 H = —571.6kJ·molˉl
H = —571.6kJ·molˉl
C.2H2+O2 = 2H2O
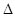 H = —571.6lkJ·molˉl
H = —571.6lkJ·molˉl
D.H2(g)+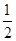 O 2(g) = H2O(1)
O 2(g) = H2O(1) 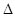 H = +285.8kJ·molˉl
H = +285.8kJ·molˉl
查看习题详情和答案>>
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是( )
A.2H2(g)+
O 2(g) = 2H2O(1) 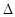 H =
—142.9kJ·molˉl
H =
—142.9kJ·molˉl
B.2H2(g)+ O 2(g) =
2H2O(1) 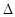 H =
—571.6kJ·molˉl
H =
—571.6kJ·molˉl
C.2H2+O2 = 2H2O
 H =
—571.6lkJ·molˉl
H =
—571.6lkJ·molˉl
D.H2(g)+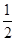 O
2(g) = H2O(1)
O
2(g) = H2O(1)  H =
+285.8kJ·molˉl
H =
+285.8kJ·molˉl
查看习题详情和答案>>
 H =" " —142.9kJ·molˉl
H =" " —142.9kJ·molˉl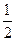 O 2(g) = H2O(1)
O 2(g) = H2O(1)