摘要: 已知:+O2(g)=== ZnO(s).ΔH= -348.3 kJ·mol-1. + O2(g)=== Ag2O(s).ΔH= -31.0 kJ·mol-1. 则Zn(s)+ Ag2O(s) === ZnO的ΔH等于( ) A.-379.3 kJ·mol-1 B.-317.3 kJ·mol-1 C.-332.8 kJ·mol-1 D.317.3 kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_298046[举报]
|
已知:(1)Zn(s)+ (2)2Ag(s)+ 则 Zn(s)+Ag2O(s) | |
| [ ] | |
A. |
-379 .3 kJ·mol-1 |
B. |
-317 .3 kJ·mol-1 |
C. |
-332 .8 kJ·mol-1 |
D. |
317 .3 kJ·mol-1 |
6.已知:(1)Zn(s)+1/2O2(g)==ZnO(s),ΔH=-348.3kJ/mol
(2) 2Ag(s)+1/2 O2(g)== Ag2O(s),ΔH=-31.0kJ/mol
则Zn(s)+ Ag2O(s)== ZnO(s)+ 2Ag(s)的ΔH等于
A.-317.3kJ/mol B.-379.3kJ/mol C.-332.8 kJ/mol D.317.3 kJ/mol
查看习题详情和答案>> 已知:(1)Zn(s)+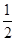 O2(g)=== ZnO(s),ΔH= -348.3 kJ·mol-1,
O2(g)=== ZnO(s),ΔH= -348.3 kJ·mol-1,
(2)2Ag(s)+
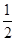 O2(g)=== Ag2O(s),ΔH= -31.0 kJ·mol-1,则Zn(s)+ Ag2O(s) === ZnO(s)+ 2Ag(s)的ΔH等于(
)
O2(g)=== Ag2O(s),ΔH= -31.0 kJ·mol-1,则Zn(s)+ Ag2O(s) === ZnO(s)+ 2Ag(s)的ΔH等于(
)
A.-317.3 kJ·mol-1 B.-379.3 kJ·mol-1 C.-332.8 kJ·mol-1 D.317.3 kJ·mol-1
查看习题详情和答案>>
 O2(g)=== ZnO(s),ΔH1= -348.3 kJ·mol-1,
O2(g)=== ZnO(s),ΔH1= -348.3 kJ·mol-1,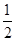 O2(g)=== ZnO(s),ΔH1= -348.3 kJ·mol-1,
O2(g)=== ZnO(s),ΔH1= -348.3 kJ·mol-1,