摘要: 已知热化学方程式: ① C2H2(g) +O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ•mol-1 ② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ•mol-1 ③ H2(g)+ O2(g) == H2O(1) △H3 = -285.8 kJ·mol-1 则反应④ 2C(s)+ H2(g) == C2H2 A. +228.2 kJ·mol-1 B. -228.2 kJ·mol-1 C. +1301.0 kJ·mol-1 D. +621.7 kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_297004[举报]
已知热化学方程式:
① C2H2(g) + O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ 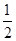 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) +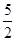 O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+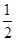 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
① C2H2(g) +
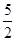 O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+
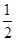 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1则反应④ 2C(s)+ H2(g) == C2H2(g)的△H为( )
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) +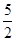 O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
① C2H2(g) +
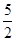 O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知热化学方程式:
① C2H2(g) + O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
则反应④ 2C(s)+ H2(g) ="=" C2H2(g)的△H为
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
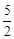 O2(g)=2CO2(g)+H2O(g)
O2(g)=2CO2(g)+H2O(g) 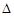 H=-1256
kJ·mol-1。下列说法正确的是( )
H=-1256
kJ·mol-1。下列说法正确的是( )