摘要:115.将0.2的CH3COONa溶液与0.1的HCl溶液等体积混合后.溶液中下列微粒的物质的量浓度的关系中.正确的是 A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH] B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+] C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH] D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+]
网址:http://m.1010jiajiao.com/timu3_id_246344[举报]
将0.2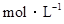 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1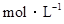 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH]
B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+]
C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH]
D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+]
查看习题详情和答案>>
将0.2的CH3COONa溶液与0.1的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
[ ]
A.c(CH3COO-)= c(Cl-)= c(H+)> c(CH3COOH )
B.c(CH3COO-)= c(Cl-)> c(CH3COOH)> c(H+)
C.c(CH3COO-) > c(Cl-) > c(H+) > c(CH3COOH)
D.c(CH3COO-)> c(Cl-)> c(CH3COOH)> c(H+)
查看习题详情和答案>>
B.c(CH3COO-)= c(Cl-)> c(CH3COOH)> c(H+)
C.c(CH3COO-) > c(Cl-) > c(H+) > c(CH3COOH)
D.c(CH3COO-)> c(Cl-)> c(CH3COOH)> c(H+)
将0.2![]() 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1![]() 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
A.[CH3COO-] = [Cl-] = [H+]> [CH3COOH]
B.[CH3COO-] = [[Cl-] >[CH3COO-] > [H+]
C.[CH3COO-] > [Cl-] >[H+] > [ CH3COOH]
D.[CH3COO-] > [Cl-] >[ CH3COOH] > [H+]
查看习题详情和答案>>
将0.2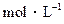 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1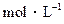 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
| A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH] |
| B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+] |
| C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH] |
| D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+] |
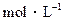 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1