网址:http://m.1010jiajiao.com/timu3_id_243246[举报]
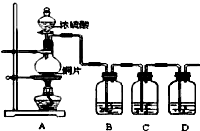 某化学兴趣小组的同学为探究二氧化硫的化学性质,设计了如图所示的装置.
某化学兴趣小组的同学为探究二氧化硫的化学性质,设计了如图所示的装置.请回答下列问题.
(1)铜和浓硫酸反应的化学方程式为
| ||
| ||
(2)B瓶中盛有品红溶液,观察到品红溶液褪色,这是因为 SO2具有
A.氧化性 B.还原性 C.漂白性
(3)D瓶中盛有NaOH溶液,作用是
(4)充分反应后,小组同学发现铜和硫酸都有剩余.若想使剩余的铜片溶解,可再加入(多选)
A.HNO3 B.NaNO3 C.Fe2O3 D.Na2CO3.
(1)浓盐酸在反应中显示出来的性质是
①只有还原性 ②还原性和酸性 ③只有氧化性 ④氧化性和酸性
(2)产生0.1mol Cl2转移电子的物质的量为
(3)ClO2具有很强的氧化性.因此,常被用作消毒剂,其消毒的效率(以单位质量得到的电子数表示)是Cl2的
| 71 |
| 27 |
| 71 |
| 27 |
某化学兴趣小组的同学为探究二氧化硫的化学性质,设计了如下图所示的装置。 请回答下列问题。
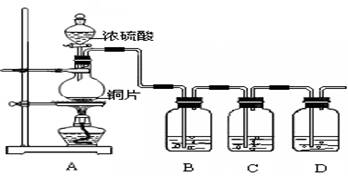
(1)铜和浓硫酸反应中浓硫酸的作用为 (A、强氧化剂 B、强还原剂)。
(2)B瓶中盛有品红溶液,观察到品红溶液褪色,这是因为 SO2具有 ,C瓶中盛有新制的氯水,观察到氯水褪色,这是因为SO2具有 。(填选项的字母)
A.氧化性 B.还原性 C.漂白性
(3)D瓶中盛有足量NaOH溶液以除去SO2, 该反应的主要产物的化学式为 。
(4)充分反应后,小组同学发现铜和硫酸都有剩余。若想使剩余的铜片溶解,可再加入
(填选项的字母)。
A.HNO3 B.NaNO3 C.NaHCO3 D.Na2CO3
查看习题详情和答案>>
(16分)将6.4 g 铜加入到50 mL a mol/L的硝酸溶液中,铜全部溶解,得到NO2和NO的混合气体。将反应后溶液稀释至100 mL,测得NO3-的浓度为3 mol/L。试求:
(1)在铜与浓硝酸反应中,硝酸表现出 ;
A.酸性 B.氧化性 C.酸性和氧化性 D.还原性
(2)铜与稀硝酸反应的离子方程式为 ;
(3)反应中生成硝酸铜的物质的量是 mol;
(4)混合气体中NO2和NO的总物质的量为 mol(用含a的式子表示);
(5)稀释后的溶液中H+的物质的量浓度为 mol/L;
(6)若a=9,则混合气体中NO2的质量为 g;
(7)用NaOH溶液吸收产生的混合气体,原理为:
2NO2+2NaOH=NaNO2+NaNO3+H2O; NO2+NO+2NaOH=2NaNO2+H2O
若生成的混合气体能被NaOH溶液完全吸收,则a的取值范围为 。
查看习题详情和答案>>
(16分)将6.4 g 铜加入到50mL a mol/L的硝酸溶液中,铜全部溶解,得到NO2和NO的混合气体。将反应后溶液稀释至100 mL,测得NO3-的浓度为3 mol/L。试求:
(1)在铜与浓硝酸反应中,硝酸表现出 ;
A.酸性 B.氧化性 C.酸性和氧化性 D.还原性
(2)铜与稀硝酸反应的离子方程式为 ;
(3)反应中生成硝酸铜的物质的量是 mol;
(4)混合气体中NO2和NO的总物质的量为 mol(用含a的式子表示);
(5)稀释后的溶液中H+的物质的量浓度为 mol/L;
(6)若a=9,则混合气体中NO2的质量为 g;
(7)用NaOH溶液吸收产生的混合气体,原理为:
2NO2+2NaOH=NaNO2+NaNO3+H2O; NO2+NO+2NaOH=2NaNO2+H2O
若生成的混合气体能被NaOH溶液完全吸收,则a的取值范围为 。
查看习题详情和答案>>