��Ŀ����
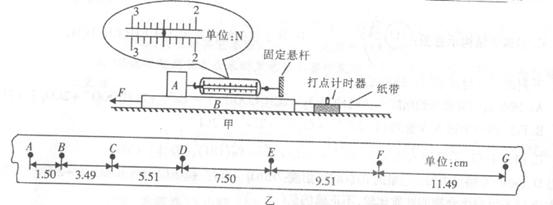
��1������һ�ο���̽����У�ijͬѧ��ͼ����ʾװ�ò�������ˮƽ�⻬�����ϵ�����A�볤������B��Ķ�Ħ���������Ѳ������A������Ϊlkg��������B������Ϊ0.5kg����ˮƽ��F������������B��ʹ�������˶����ȶ�ʱ���ɳ�ʾ����ͼ��ʾ���ѷŴ���A��B�ʵĶ�Ħ��������=
�ڽ�ֽ�������ڽ�����B�ĺ��棬ͨ������ʱ����������һЩ��ʱ�㣬������Ľ����ͼ����ʾ��ͼ�и���������ʱ����Ϊ0.1s���ɴ˿�֪ˮƽ��F=

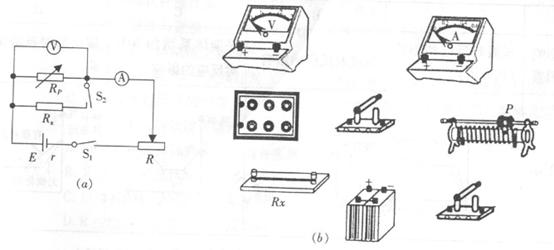
��2��ijͬѧ���������IJ���һ����˿�ĵ���Rx����ԴE���ʵ����̵ĵ���������ѹ����һֻ��������������R������Rp������S1��S2���������ɣ�����Ƶĵ�·ͼ��ͼ��a����ʾ��
ʵ�鲽�裺����S1�պϣ�S2�Ͽ�������R��Rp��ʹ�������͵�ѹ��ʾ����������������ʾ��ΪI1��U1���ٱ���R��Rp��ֵ���䣬�պ�S2���µ������͵�ѹ��ʾ��ΪI2��U2��
���밴��·ͼ��ʵ��ͼ��b��-E���ߣ�Ҫ���̨S1ǰ�������������Ļ�����ͷP������ȷ��λ�ã���
����������Rx=
���õ����ʾ����ʾ����
�۴�ʵ����������������裬��ѹ�����費��������������ֵ
����ͼ��a����·��
0.25
0.25
����gȡ10m/s���ڽ�ֽ�������ڽ�����B�ĺ��棬ͨ������ʱ����������һЩ��ʱ�㣬������Ľ����ͼ����ʾ��ͼ�и���������ʱ����Ϊ0.1s���ɴ˿�֪ˮƽ��F=
3.5
3.5
N��
��2��ijͬѧ���������IJ���һ����˿�ĵ���Rx����ԴE���ʵ����̵ĵ���������ѹ����һֻ��������������R������Rp������S1��S2���������ɣ�����Ƶĵ�·ͼ��ͼ��a����ʾ��
ʵ�鲽�裺����S1�պϣ�S2�Ͽ�������R��Rp��ʹ�������͵�ѹ��ʾ����������������ʾ��ΪI1��U1���ٱ���R��Rp��ֵ���䣬�պ�S2���µ������͵�ѹ��ʾ��ΪI2��U2��
���밴��·ͼ��ʵ��ͼ��b��-E���ߣ�Ҫ���̨S1ǰ�������������Ļ�����ͷP������ȷ��λ�ã���
����������Rx=
| U1U2 |
| U1I2-U21 |
| U1U2 |
| U1I2-U21 |
�۴�ʵ����������������裬��ѹ�����費��������������ֵ
����
����
��ʵֵ������ڡ�����С�ڡ����ڡ���������ͼ��a����·��
��
��
�����ѹ�������裨����ܡ����ܡ�������������1�����ȶ�ʱ��A���ܵĻ���Ħ�������ڵ��ɵ������ݴ��з��̿�����ȷ���
��2�����������B�ļ��ٶȴ�С��Ȼ�����ţ�ٵڶ�����F-mAg��=mBa�����������������С��
��3����ʵ���У���Ч�Ŀ˷������ڵ�ѹ���ķ�����ɵ������ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪR1=
�����ݵڶ������������Rp����ѹ����Rx�IJ���ֵΪR2=
���ɴ˸��ݲ�����·֪ʶ�������R�ĵ���Rx�Լ���ѹ���������С��
��2�����������B�ļ��ٶȴ�С��Ȼ�����ţ�ٵڶ�����F-mAg��=mBa�����������������С��
��3����ʵ���У���Ч�Ŀ˷������ڵ�ѹ���ķ�����ɵ������ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪR1=
| U1 |
| I1 |
| U2 |
| I2 |
����⣺��1���ٵ��ɳӵĶ���ΪF=2.50N�����ݶ���ƽ��֪ʶ�У�F=mAg��
�ɴ˿ɽ�ã���=0.25��
�ʴ�Ϊ��0.25��
�ڸ����ȱ���ֱ���˶������ۡ�x=aT2���У�
x6-x3=3a1T2 ��
x5-x2=3a2T2 ��
x4-x1=3a3T2 ��
a=
��
�����٢ڢۢܽ�ã�a=
��
�������ݽ�ã�a=2.00m/s2��
����ţ�ٵڶ������У�F-mAg��=mBa���ɴ˽��ΪF=3.5N
�ʴ�Ϊ��3.5��
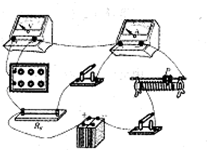
��2����ʵ��ͼ������ͼ��ʾ��
 �ڸ��ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪ��
�ڸ��ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪ��
R1=
��
���ݵڶ������������Rp����ѹ����Rx�IJ���ֵΪ��
R2=
��
���ݲ�����·�ص�Ϊ��R2=
��
�����٢ڢ۽�ã�Rx=
�۸�ʵ���У���Ч�Ŀ˷������ڵ�ѹ���ķ�����ɵ�ϵͳ����˱���������ֵ������ʵֵ��
�ܸ��ݵ�·�IJ���֪ʶͬ�����������ѹ�������裮
�ʴ�Ϊ���ټ���ͼ����
���۵��ڣ����ܣ�
�ɴ˿ɽ�ã���=0.25��
�ʴ�Ϊ��0.25��
�ڸ����ȱ���ֱ���˶������ۡ�x=aT2���У�
x6-x3=3a1T2 ��
x5-x2=3a2T2 ��
x4-x1=3a3T2 ��
a=
| a1+a2+a3 |
| 3 |
�����٢ڢۢܽ�ã�a=
| (x4+x5+x6)-(x3+x2+x1) |
| 9T2 |
�������ݽ�ã�a=2.00m/s2��
����ţ�ٵڶ������У�F-mAg��=mBa���ɴ˽��ΪF=3.5N
�ʴ�Ϊ��3.5��
��2����ʵ��ͼ������ͼ��ʾ��
 �ڸ��ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪ��
�ڸ��ݵ�һ�β����������Լ����Rp��ѹ����������ֵΪ��R1=
| U1 |
| I1 |
���ݵڶ������������Rp����ѹ����Rx�IJ���ֵΪ��
R2=
| U2 |
| I2 |
���ݲ�����·�ص�Ϊ��R2=
| R1Rx |
| R1+Rx |
�����٢ڢ۽�ã�Rx=
| U1U2 |
| U1I2-U2I1 |
�۸�ʵ���У���Ч�Ŀ˷������ڵ�ѹ���ķ�����ɵ�ϵͳ����˱���������ֵ������ʵֵ��
�ܸ��ݵ�·�IJ���֪ʶͬ�����������ѹ�������裮
�ʴ�Ϊ���ټ���ͼ����
| U1U2 |
| U1I2-U2I1 |
�����������ǵ�ѧʵ�黹����ѧʵ�飬��ȷʵ��ԭ�����ǽ��ʵ������Ĺؼ���ͬʱҪ����Ӧ����ѧ��������֪ʶ���ʵ�����⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ��gȡ10m/s2��
��gȡ10m/s2�� ��gȡ10m/s2��
��gȡ10m/s2��