��Ŀ����
��ͼ��ʾ������ԴS���Բ��ϵز�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ư��һ��ˮƽ���ҵļ��ٵ糡�����ٲ��ƣ����پ�С��O2��ֱ�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ���߳�ΪL�ij�����ABCDEFGH������ǿ�ų��У��Ÿ�Ӧǿ�ȴ�СΪB2��������ADHEƽ�洹ֱ����Aָ��B����������HFC��һ�������ε�Ӳ�����ϰ壬��ͼ��ʾ��
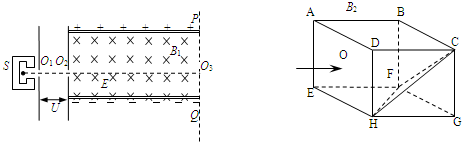
��1����Ҫ����Դ�������������O2O3ֱ���˶�������ֱ��PQ�������Ÿ��ϳ������ٵ�ѹUӦ���
��2��������Ӵ�O3�������Ÿ��ϳ����ٴ�ֱADHEƽ�沢�Ӹ�ƽ���������O�����볤�����У�ǡ����HFC������Ӳ�����ϰ��CF�����������������������������ٶȴ�С���䣬����仯���ع�ķ��䶨�ɣ���ô��Ҫ������巢����һ����ײ�������BCGFƽ��ˮƽ��������壬��Ÿ�Ӧǿ��B2ӦΪ�����Ҫ������巢����һ����ײ���ܴ�ABFEƽ����������壬��Ÿ�Ӧǿ��B2����Ӧ���
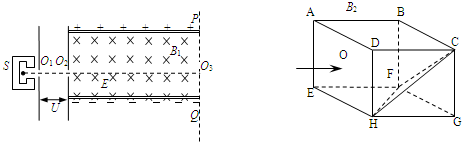
��1����Ҫ����Դ�������������O2O3ֱ���˶�������ֱ��PQ�������Ÿ��ϳ������ٵ�ѹUӦ���
��2��������Ӵ�O3�������Ÿ��ϳ����ٴ�ֱADHEƽ�沢�Ӹ�ƽ���������O�����볤�����У�ǡ����HFC������Ӳ�����ϰ��CF�����������������������������ٶȴ�С���䣬����仯���ع�ķ��䶨�ɣ���ô��Ҫ������巢����һ����ײ�������BCGFƽ��ˮƽ��������壬��Ÿ�Ӧǿ��B2ӦΪ�����Ҫ������巢����һ����ײ���ܴ�ABFEƽ����������壬��Ÿ�Ӧǿ��B2����Ӧ���
��������1�����������ܵ��ĵ糡��������������ƽ�⣬��֪�ٶȴ�С�����ɶ��ܶ�����������⣻
��2���������������ṩ����������ţ�ٵڶ����ɣ��������Ÿ�Ӧǿ�ȣ������ݼ��ι�ϵ����������ܴ�ABFEƽ�����������ĴŸ�Ӧǿ�ȣ�
��2���������������ṩ����������ţ�ٵڶ����ɣ��������Ÿ�Ӧǿ�ȣ������ݼ��ι�ϵ����������ܴ�ABFEƽ�����������ĴŸ�Ӧǿ�ȣ�
����⣺��1�����������������ǿ�糡����ǿ�ų���������ֱ���˶�����
qvB1=qE
��v=
�����ڼ��ٵ糡�м���ʱ���ɶ��ܶ����ã�
qU=
mv2
U=
=
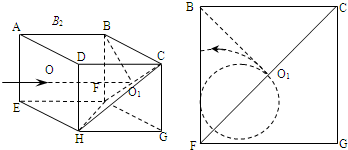
��2��������CF�ߵ��е�O1�����ϰ���ײ����BCGFƽ������O1B����������BCGFƽ��������Բ���˶���
��Ҫ�����ܴ�ABFE���ڵ�ƽ��ˮƽ����������壬���ӵ��˶��켣����BF��ֱ��
��������Բ���˶���Բ�ı���F�㣬Բ���˶��İ뾶R=
CF=
L��
��ţ�ٵڶ����ɵã�qvB2=m
����B2=
��� B2=
��Ҫ���Ӳ��ܴ�ABFE���ڵ�ƽ������������壬��ͼ��ʾ����������Բ���˶���Բ�ı���O1F�ϣ���Բ���˶��İ뾶Ϊr�� ��
r+rcos45��=
��r=
ͬ�����B2=
����B2����Ϊ
�𣺣�1����Ҫ����Դ�������������O2O3ֱ���˶�������ֱ��PQ�������Ÿ��ϳ������ٵ�ѹUӦ��
��2��������Ӵ�O3�������Ÿ��ϳ����ٴ�ֱADHEƽ�沢�Ӹ�ƽ���������O�����볤�����У�ǡ����HFC������Ӳ�����ϰ��CF�����������������������������ٶȴ�С���䣬����仯���ع�ķ��䶨�ɣ���ô��Ҫ������巢����һ����ײ�������BCGFƽ��ˮƽ��������壬��Ÿ�Ӧǿ�� B2=
����Ҫ������巢����һ����ײ���ܴ�ABFEƽ����������壬��Ÿ�Ӧǿ��B2����ӦΪ
��
qvB1=qE
��v=
| E |
| B1 |
�����ڼ��ٵ糡�м���ʱ���ɶ��ܶ����ã�
qU=
| 1 |
| 2 |
U=
| mv2 |
| 2q |
| mE2 | ||
2q
|
��2��������CF�ߵ��е�O1�����ϰ���ײ����BCGFƽ������O1B����������BCGFƽ��������Բ���˶���
��Ҫ�����ܴ�ABFE���ڵ�ƽ��ˮƽ����������壬���ӵ��˶��켣����BF��ֱ��
��������Բ���˶���Բ�ı���F�㣬Բ���˶��İ뾶R=
| 1 |
| 2 |
| ||
| 2 |
��ţ�ٵڶ����ɵã�qvB2=m
| v2 |
| R |
����B2=
| mv |
| qR |
��� B2=
| ||
| qB1L |
��Ҫ���Ӳ��ܴ�ABFE���ڵ�ƽ������������壬��ͼ��ʾ����������Բ���˶���Բ�ı���O1F�ϣ���Բ���˶��İ뾶Ϊr�� ��
r+rcos45��=
| L |
| 2 |
��r=
| L | ||
2+
|
ͬ�����B2=
(2+
| ||
| qB1L |
(2+
| ||
| qB1L |

�𣺣�1����Ҫ����Դ�������������O2O3ֱ���˶�������ֱ��PQ�������Ÿ��ϳ������ٵ�ѹUӦ��
��2��������Ӵ�O3�������Ÿ��ϳ����ٴ�ֱADHEƽ�沢�Ӹ�ƽ���������O�����볤�����У�ǡ����HFC������Ӳ�����ϰ��CF�����������������������������ٶȴ�С���䣬����仯���ع�ķ��䶨�ɣ���ô��Ҫ������巢����һ����ײ�������BCGFƽ��ˮƽ��������壬��Ÿ�Ӧǿ�� B2=
| ||
| qB1L |
(2+
| ||
| qB1L |
�����������еĸ��ϳ������ٶ�ѡ��Ĺ��ܣ�����ų�������ݶ���ѧ������ȷ���˶��켣���ٽ��м��㣮

��ϰ��ϵ�д�
�����Ŀ
 ��2010?Խ������ģ����ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45�㣮��������ͼ������O2O3���ӳ��߽���PQ��MN֮�������
��2010?Խ������ģ����ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45�㣮��������ͼ������O2O3���ӳ��߽���PQ��MN֮�������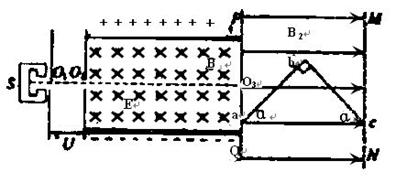

 ��ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45°����������ͼ������O2O3���ӳ��߽���PQ��MN֮�������
��ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45°����������ͼ������O2O3���ӳ��߽���PQ��MN֮������� ��ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45°����������ͼ������O2O3���ӳ��߽���PQ��MN֮�������
��ͼ��ʾ������ԴS���Բ��ϲ�������Ϊm�������Ϊ+q�����ӣ��������ƣ������Ӵ�O1��Ʈ��һ��ˮƽ����ļ��ٵ糡�����ٲ��ƣ����پ���С��O2�������������ǿ�糡����ǿ�ų����糡ǿ�ȴ�СΪE���Ÿ�Ӧǿ�ȴ�СΪB1��������ͼ������PQ��MN֮�������ˮƽ���ҵ���ǿ�ų����ų���Χ�㹻�Ÿ�Ӧǿ�ȴ�СΪB2��һ���۳�ֱ�ǵ�Ӳ������Ƭabc�������磬���Ⱥͺ�ȶ���С�ɺ��ԣ�������PQ��MN֮�䣬����ͼ��ͼ��a��c����ֱ�λ��PQ��MN�ϣ�ab=bc=L��a=45°����������ͼ������O2O3���ӳ��߽���PQ��MN֮�������