��Ŀ����
�ڡ��ⶨ�����ĵ����ʡ�ʵ���У����ò�����������У���������˿�����·���ֵij���ԼΪ50cm��
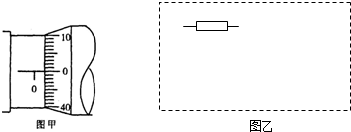
��1��������������������˿��ֱ��������ijһ�β��������ͼ1��ʾ�������Ӧ
Ϊ mm����ֵ�ӽ���β�����ƽ��ֵ��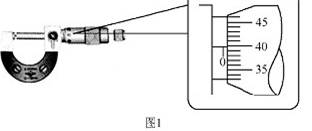
��2���÷����������˿�ĵ���Rx��ʵ����������Ϊ������裨�綯��3V������Լ1������������������Լ0.1��������ѹ��������Լ3k����������������R��0��20���������2A�������ء��������ɡ�
ijС��ͬѧ��������������ȷ���Ӻõ�·������ʵ���������¼�������£�
| ���� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| U/V | 0.10 | 0.30 | 0.70 | 1.00 | 1.50 | 1.70 | 2.30 |
| I/A | 0.020 | 0.060 | 0.160 | 0.220 | 0.340 | 0.460 | 0.520 |
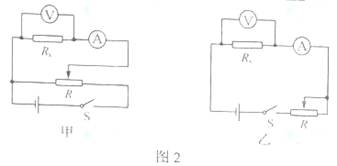
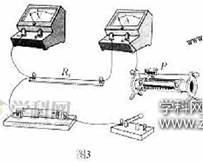
��3��ͼ3�Dz���Rx��ʵ������ʵ��ͼ��ͼ���������˲��ֵ��ߣ������������Ļ�ƬP���ڱ�������һ�ˣ�����ݣ�2����ѡ�ĵ�·ͼ���������ͼ3��ʵ�������ߣ���ʹ�պϿ��ص�˲�䣬��ѹ��������������ڱ��ջ���
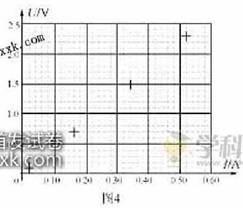
��4�����С���ͬѧ������ֽ�Ͻ���U��I����ϵ����ͼ4��ʾ��ͼ���ѱ������������ݶ�Ӧ��4������㡣����ͼ4�б����2��4��6�β������ݵ�����㣬������U-Iͼ�ߣ���ͼ�ߵõ�����˿����ֵ Rx= ����������λ��Ч���֣���
��5�������������ݿ��Թ��������˿�ĵ�����ԼΪ ����ѡ��ǰ�ķ��ţ���
A��1��
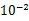 ����m B��1��
����m B��1��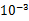 ����m
����m C��1��
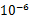 ����m D��1��
����m D��1��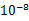 ����m
����m��6���κ�ʵ���������������ʵ�����ò�����������У�����й�������˵������ȷ��ѡ���� ���ж����ȷѡ���
A��������������������ֱ��ʱ�����ڶ���������������ϵͳ���
B�����ڵ������͵�ѹ������������������żȻ���
C�������������͵�ѹ��������������ڣ������������ڲ����DZ������ϵͳ���
D����U��Iͼ�������������������Լ�СżȻ���
��1��0.398(0.395~0.399)
��2����
��3����ͼ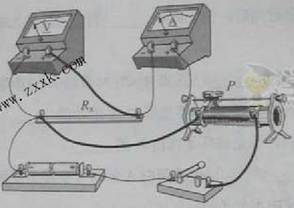
��4����ͼ����4.3��4.7��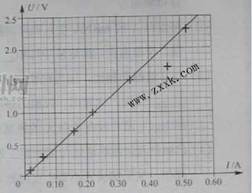
��5��C
��6��CD
����

��ϰ��ϵ�д�
�����Ŀ
 �ڡ��ⶨ�����ĵ����ʡ���ʵ���У��ⶨ��ֵԼΪ3-5���Ľ���˿�ĵ����ʣ�ʵ�������õĵ�ѹ���������0-3V������3k�����������������0-0.6A������0.1������������һЩ���ģ�
�ڡ��ⶨ�����ĵ����ʡ���ʵ���У��ⶨ��ֵԼΪ3-5���Ľ���˿�ĵ����ʣ�ʵ�������õĵ�ѹ���������0-3V������3k�����������������0-0.6A������0.1������������һЩ���ģ�