��Ŀ����
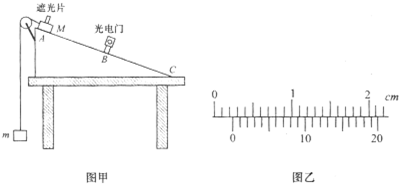
4�� ��ͼ��ʾ������ֱƽ���ڣ��⻬��Եֱ��AC��뾶ΪR��Բ�ܽ���B��C���㣬��Բ�Ĵ���һ�̶��������ɣ�BΪAC���е㣬C��λ��Բ�ܵ���͵㣮����һ����Ϊm�������Ϊq������ֱ���ϵĴ�����С���A���ɾ�ֹ��ʼ�ظ��»�����֪�������ٶ�Ϊg��A����C���ˮƽ�����ֱ�߶�Ϊ3R��С��B��ʱ���ٶȴ�СΪ2$\sqrt{gR}$��
��ͼ��ʾ������ֱƽ���ڣ��⻬��Եֱ��AC��뾶ΪR��Բ�ܽ���B��C���㣬��Բ�Ĵ���һ�̶��������ɣ�BΪAC���е㣬C��λ��Բ�ܵ���͵㣮����һ����Ϊm�������Ϊq������ֱ���ϵĴ�����С���A���ɾ�ֹ��ʼ�ظ��»�����֪�������ٶ�Ϊg��A����C���ˮƽ�����ֱ�߶�Ϊ3R��С��B��ʱ���ٶȴ�СΪ2$\sqrt{gR}$����1����С����C��ʱ���ٶȴ�С��
��2����A��B�����ĵ��Ʋ�UAB��
��3������C��Ϊ����Ƶ㣬��ȷ��A��ĵ��ƣ�
���� ��1��BΪAC���е㣬��BC��AB��ĸ߶Ȳ��Ϊ1.5R����B��C�������ö��ܶ�����ʽ���C����ٶȣ�
��2����A��B�������ö��ܶ�����ʽ���A��B����ĵ��Ʋ�UAB��
��3��B��C������ͬһ���������ϣ��ʵ�����ȣ���B�����ҲΪ�㣬����UAB=��A-��B���A��ĵ��ƣ�
��� �⣺��1��С����A��B���̣��ɶ��ܶ�����
$mg•\frac{3}{2}R+q{U}_{AB}=\frac{1}{2}m��2\sqrt{gR}��^{2}$����
С����A��C���̣��ɶ��ܶ�����
mg3R+qUAC=$\frac{1}{2}m{{v}_{c}}^{2}$ ��
����UAB=UAC ��
�ɢ٢ڢ�ʽ�ɵ�С����C��ʱ���ٶȴ�СΪvC=$\sqrt{7gR}$��
��2���ɢ�ʽ�ɵ�A��B�����ĵ��Ʋ�
UAB=-$\frac{mgR}{2q}$
��3��B��C������ͬһ���������ϣ��ʵ�����ȣ���B�����ҲΪ�㣬����UAB=��A-��B
��A=$-\frac{mgR}{2q}$��
�𣺣�1��С����C��ʱ���ٶȴ�С��$\sqrt{7gR}$��
��2��A��B�����ĵ��Ʋ�Ϊ-$\frac{mgR}{2q}$��
��3��A��ĵ���Ϊ$-\frac{mgR}{2q}$��
���� ����ؼ����ݶ��ܶ�����ʽ���������������Բ��Ϊ�����棬ץסС���A��C��A��B�糡��������ȣ����ö��ܶ���������⣬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Ϊ���о��˶�Ա�ļ����������ɽ����ڱ������˶�Ա��Ϊ�ʵ� | |
| B�� | �˶�Ա����������У���о���ˮ�����ȼ������� | |
| C�� | ǰһ��λ���õ�ʱ��̣���һ��λ���õ�ʱ�䳤 | |
| D�� | ǰһ��ʱ����λ�ƴ�һ��ʱ����λ��С |
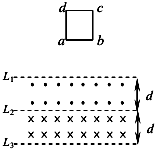 ��ͼ��ʾ������Ϊd������ˮƽ����L1��L2��L2��L3֮��ֱ��д�ֱֽ�����⡢�������ǿ�ų����Ÿ�Ӧǿ�ȴ�С��ΪB��һ���߳�Ҳ��d�������ε��߿�L1�Ϸ�һ���ߴ��ɾ�ֹ��ʼ�������䣬��ab�߸�Խ��L1����ų�ʱ��ǡ�����ٶ�v1������ֱ���˶�����ab����Խ��L2�˶���L3֮ǰ��ij��ʱ�̣��߿��ֿ�ʼ���ٶ�v2������ֱ���˶������߿�ӽ���ų����ٶȱ�Ϊv2�Ĺ����У����߿�Ķ��ܱ仯����СΪ��Ek���������߿�������СΪW1�����������߿�������СΪW2���ù��̵��߿��в����ĵ��ܴ�СΪE0������˵������ȷ���ǣ�������
��ͼ��ʾ������Ϊd������ˮƽ����L1��L2��L2��L3֮��ֱ��д�ֱֽ�����⡢�������ǿ�ų����Ÿ�Ӧǿ�ȴ�С��ΪB��һ���߳�Ҳ��d�������ε��߿�L1�Ϸ�һ���ߴ��ɾ�ֹ��ʼ�������䣬��ab�߸�Խ��L1����ų�ʱ��ǡ�����ٶ�v1������ֱ���˶�����ab����Խ��L2�˶���L3֮ǰ��ij��ʱ�̣��߿��ֿ�ʼ���ٶ�v2������ֱ���˶������߿�ӽ���ų����ٶȱ�Ϊv2�Ĺ����У����߿�Ķ��ܱ仯����СΪ��Ek���������߿�������СΪW1�����������߿�������СΪW2���ù��̵��߿��в����ĵ��ܴ�СΪE0������˵������ȷ���ǣ�������| A�� | �ڵ��߿���������У�����������������������v2��v1 | |
| B�� | �ù������߿��ܵı仯����СΪ��Ek=W2-W1-E0 | |
| C�� | �ù������߿��еĵ�������û�з����仯 | |
| D�� | �ڵ��߿�ͨ���ų������������У��߿��е�ƽ����Ӧ����Ϊ�� |
| A�� | $\frac{{2\sqrt{hR}}}{t}$ | B�� | $\frac{{\sqrt{2hR}}}{t}$ | C�� | 11.2km/s | D�� | 7.9km/s |
| A�� | Ek=hv-W | B�� | Ek=hv+W | C�� | W=Ek-hv | D�� | W=Ek+hv |
 ��ͼ��ʾ�����м�г�Შ�ֱ���x�������������������Դ�ֱ�λ��x=-0.2m��x=1.2m�������в����ٶȴ�С��Ϊv=0.4m/s������Դ�������ΪA=2cm��ͼʾΪt=0ʱ�����в���ͼ����������ͼ��ʾ������ʱ��ƽ��λ��λ��x=0.2m��x=0.8m��P��Q���ʵ�տ�ʼ���ʵ�M��ƽ��λ�ô���x=0.5m�������ڸ��ʵ��˶�������ж���ȷ���ǣ�������
��ͼ��ʾ�����м�г�Შ�ֱ���x�������������������Դ�ֱ�λ��x=-0.2m��x=1.2m�������в����ٶȴ�С��Ϊv=0.4m/s������Դ�������ΪA=2cm��ͼʾΪt=0ʱ�����в���ͼ����������ͼ��ʾ������ʱ��ƽ��λ��λ��x=0.2m��x=0.8m��P��Q���ʵ�տ�ʼ���ʵ�M��ƽ��λ�ô���x=0.5m�������ڸ��ʵ��˶�������ж���ȷ���ǣ�������| A�� | t=0ʱ���ʵ�P��Q����y���������˶� | |
| B�� | t=1sʱ�̣��ʵ�M��λ��Ϊ-4cm | |
| C�� | t=1sʱ�̣��ʵ�M��λ��Ϊ+4cm | |
| D�� | t=0.75sʱ�̣��ʵ�P��Q���˶���x=0.5m |