��Ŀ����
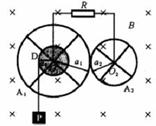
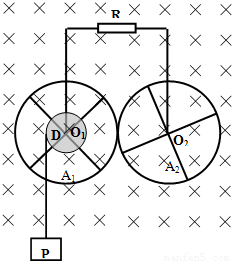
��ͼ��ʾ������������A1��A2������ͨ���������IJ������洹ֱ�Ĺ̶��Ĺ⻬����ϸ��O1��O2ת����O1��O2�ƽ�У�ˮƽ���ã�ÿ�����������ĸ����������ͽ�������ɣ�A1�ֵķ�����Ϊa1������ΪR1��A2�ֵķ�����ҲΪa1������ΪR2�����ӷ����Ľ������Ŀ�������趼���Ժ��ԣ��뾶Ϊa0�ľ�ԵԲ��D��A1ͬ���ҹ�����һ��һ��ϸ����һ�˹̶���D��Ե�ϵ�ij�㣬����D�����㹻����������һ����Ϊm������P����P����ʱ��ͨ��ϸ������D��A1����ת����ת��������A1��A2���ֽӴ�������Ի����������������֮�䱣�����ýӴ�������Ի��������������ϸ��֮�䱣�����õĵ�Ӵ�����ϸ��ͨ��������һ��ֵΪR�ĵ�����������R��A1��A2�����з����ĵ����⣬���н������趼���ƣ�����װ�ô��ڴŸ�Ӧǿ��ΪB����ǿ�ų��У��ų�������ת��ƽ�У��ֽ�P�ɾ�ֹ���ͷţ�
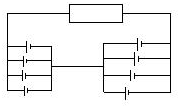
��1����ͼ�б����˸������еĵ�����������װ�õĵ�Ч��·ͼ
��2�����ɾ�ֹ��ʼ��������У�����ϵͳ�����������ת���ģ�
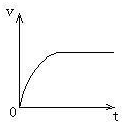
��3�����Ի��������ɾ�ֹ��ʼ��������е��ٶ�-ʱ��ͼ��
��4����P��������е�����ٶȣ�
��1����ͼ�б����˸������еĵ�����������װ�õĵ�Ч��·ͼ
��2�����ɾ�ֹ��ʼ��������У�����ϵͳ�����������ת���ģ�
��3�����Ի��������ɾ�ֹ��ʼ��������е��ٶ�-ʱ��ͼ��
��4����P��������е�����ٶȣ�

��1��P���ͷź�ϸ����������D������е���أ�����D��A1����ʱ��ļ���ת����ͨ����������֮��������˶��ĽӴ���A1����A2��˳ʱ��ļ����˶��������������ӵķ����и�ų��ߣ�������A1�������ܱ��ط���ָ����ĵ綯�ƣ���A2���������ط���ָ���ܱߵĵ綯�ƣ�������R���ɱպϵ�·��A1��A2�и������������ص綯�Ʒ���ĵ������ڴų��з����ܵ������������ѿ����������������ĵ�������������أ�ʹA1��A2����ת������ͷ������A1��A2����ʼ�ľ�ֹ״̬����ת����������֮���������������������ֱ��������������е������ȣ�D��A1��A2ֹͣ������ת���������Ƚ���ת������ʱP�������䣬
����������ߵ��������������ָ��Բ�ģ��ұߵ������������������Բ�ģ�
��Ч��·����ͼ��ʾ��
��2�����ٹ��̵�����ת������������ת��Ϊ���ܺ����ܣ�
���ٽΣ���������ת��Ϊ���ܣ�
��3�������ɾ�ֹ��ʼ��������е��ٶ�-ʱ�䣺�������ٶȼ�С�ļ����˶�����������ֱ���˶���ͼ������ͼ��ʾ��
��4�������ٶ�Ϊv����A1�Ľ��ٶ���1=
| v |
| a1 |
A1����A2ת����A2�Ľ��ٶȦ�2��A1�Ľ��ٶȦ�1֮��Ĺ�ϵΪ��1a1=��2a1��2��
A1��ÿ�����������ĸ�Ӧ�綯�ƾ�ΪE1=
| 1 |
| 2 |
| a | 21 |
�����ֱ�֮��ĵ綯�ƾ���A1�����������綯�ƵIJ���������ֵ����3��ʽ��ͬ����A2�У������ֱ�֮��ĵ綯�ƾ���A2�����������綯�ƵIJ���������ֵE2=
| 1 |
| 2 |
| a | 21 |
A1��ÿ�������ĵ���ΪR1�������ֱ�֮��ĵ綯�ƾ���A1�����������綯�ƵIJ���������ֵΪRA1=
| R1 |
| 4 |
A2��ÿ�������ĵ���ΪR2�������ֱ�֮��ĵ綯�ƾ���A2�����������綯�ƵIJ���������ֵΪRA2=
| R2 |
| 4 |
A1�֡�A2�ֺ͵���R���ɴ�����·�����еĵ���ΪI=
| E1+E2 |
| R+RA1+RA2 |
�ԣ�1������6��ʽ���루7��ʽ����I=
| ||||
R+
|
��P�����½�ʱ��������ϵͳ��˵�������Ĺ��ʵ������е���Ľ����ȹ���֮�ͣ��� mgv=I2��R+
| R1 |
| 4 |
| R2 |
| 4 |
�ԣ�8��ʽ���루9��ʽ��V=
| mg(4R+R1+R2)a02 |
| 4B2a14 |
�𣺣�1����ͼ�б����˸������еĵ�������������װ�õĵ�Ч��·ͼ������ͼ��
��2�����ɾ�ֹ��ʼ��������У�����ϵͳ�������Ǽ��ٹ��̵�����ת������������ת��Ϊ���ܺ����ܣ�
���ٽΣ���������ת��Ϊ���ܣ�
��3�����Ի��������ɾ�ֹ��ʼ��������е��ٶ�-ʱ��ͼ��������ͼ��
��4����P��������е�����ٶ�V=
| mg(4R+R1+R2)a02 |
| 4B2a14 |

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
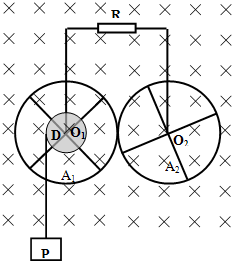 ��ͼ��ʾ������������A1��A2������ͨ���������IJ������洹ֱ�Ĺ̶��Ĺ⻬����ϸ��O1��O2ת����O1��O2�ƽ�У�ˮƽ���ã�ÿ�����������ĸ����������ͽ�������ɣ�A1�ֵķ�����Ϊa1������ΪR1��A2�ֵķ�����ҲΪa1������ΪR2�����ӷ����Ľ������Ŀ�������趼���Ժ��ԣ��뾶Ϊa0�ľ�ԵԲ��D��A1ͬ���ҹ�����һ��һ��ϸ����һ�˹̶���D��Ե�ϵ�ij�㣬����D�����㹻����������һ����Ϊm������P����P����ʱ��ͨ��ϸ������D��A1����ת����ת��������A1��A2���ֽӴ�������Ի����������������֮�䱣�����ýӴ�������Ի��������������ϸ��֮�䱣�����õĵ�Ӵ�����ϸ��ͨ��������һ��ֵΪR�ĵ�����������R��A1��A2�����з����ĵ����⣬���н������趼���ƣ�����װ�ô��ڴŸ�Ӧǿ��ΪB����ǿ�ų��У��ų�������ת��ƽ�У��ֽ�P�ɾ�ֹ���ͷţ�
��ͼ��ʾ������������A1��A2������ͨ���������IJ������洹ֱ�Ĺ̶��Ĺ⻬����ϸ��O1��O2ת����O1��O2�ƽ�У�ˮƽ���ã�ÿ�����������ĸ����������ͽ�������ɣ�A1�ֵķ�����Ϊa1������ΪR1��A2�ֵķ�����ҲΪa1������ΪR2�����ӷ����Ľ������Ŀ�������趼���Ժ��ԣ��뾶Ϊa0�ľ�ԵԲ��D��A1ͬ���ҹ�����һ��һ��ϸ����һ�˹̶���D��Ե�ϵ�ij�㣬����D�����㹻����������һ����Ϊm������P����P����ʱ��ͨ��ϸ������D��A1����ת����ת��������A1��A2���ֽӴ�������Ի����������������֮�䱣�����ýӴ�������Ի��������������ϸ��֮�䱣�����õĵ�Ӵ�����ϸ��ͨ��������һ��ֵΪR�ĵ�����������R��A1��A2�����з����ĵ����⣬���н������趼���ƣ�����װ�ô��ڴŸ�Ӧǿ��ΪB����ǿ�ų��У��ų�������ת��ƽ�У��ֽ�P�ɾ�ֹ���ͷţ�