��Ŀ����
 ��ͼ��ʾ��һ������ֱ���ã���һ����Ϊm�Ļ����ڸ��ڷ����һ�������������壬�����ĵײ���װ��һ������˿���õ��ߺ�����Դ��������֪���ױںͻ������Ǿ��ȵģ����ױ��������Ӵ��⻬�Ҳ�©�����ֽ�ͨ��Դ������˿�Ը������建�����ȣ������������ΪS��������ѹǿΪp0������˿�ȹ���ΪP�����ͨ��tʱ���ڻ������������ƶ��߶�h����
��ͼ��ʾ��һ������ֱ���ã���һ����Ϊm�Ļ����ڸ��ڷ����һ�������������壬�����ĵײ���װ��һ������˿���õ��ߺ�����Դ��������֪���ױںͻ������Ǿ��ȵģ����ױ��������Ӵ��⻬�Ҳ�©�����ֽ�ͨ��Դ������˿�Ը������建�����ȣ������������ΪS��������ѹǿΪp0������˿�ȹ���ΪP�����ͨ��tʱ���ڻ������������ƶ��߶�h����������������ѹǿ�Ĵ�С��
��tʱ�����������������Ĺ������ܵı仯����
��������ͨ��Դ������˿�Ը������建�����ȹ����У����������巢����ѹ�仯�����ݻ�������ƽ����ѹǿ�����ݹ��Ĺ�ʽ����������������ѧ��һ���ɷ���������α仯��
����⣺���ɻ�������ƽ�⣬�ڲ�����ѹǿΪP��
p0+
=p
���������������
W=pSh=p0Sh+mgh
���ܵı仯����
��E=Q-W=Pt-mgh-p0Sh
�𣺢�����������ѹǿ�Ĵ�Сp0+
��
��tʱ�����������������Ĺ�p0Sh+mgh�����ܵı仯��Pt-mgh-p0Sh��
p0+
| mg |
| S |
���������������
W=pSh=p0Sh+mgh
���ܵı仯����
��E=Q-W=Pt-mgh-p0Sh
�𣺢�����������ѹǿ�Ĵ�Сp0+
| mg |
| S |
��tʱ�����������������Ĺ�p0Sh+mgh�����ܵı仯��Pt-mgh-p0Sh��
��������������Ҫ�ж������ѹǿ���䣬������ѧ֪ʶ���⣬���Ҫ��������ѧ��һ���ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��ͼ��ʾ��һ������ֱ���ã���������һ�������ɺ��ԵĻ�������һ����������������������ڣ����������ױ���Ħ�������崦��ƽ��״̬���ֱ����¶Ȳ������������бһ�㣬�ڴﵽƽ�����ԭ����ȣ�������
��ͼ��ʾ��һ������ֱ���ã���������һ�������ɺ��ԵĻ�������һ����������������������ڣ����������ױ���Ħ�������崦��ƽ��״̬���ֱ����¶Ȳ������������бһ�㣬�ڴﵽƽ�����ԭ����ȣ�������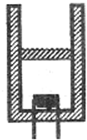 ��ѡ��ģ��3-3��
��ѡ��ģ��3-3�� ��ͼ��ʾ��һ������ֱ���ã���һ����Ϊm�Ļ����ڸ��ڷ����һ�������������壬�����ĵײ���װ��һ������˿���õ��ߺ�����Դ��������֪���ױںͻ������Ǿ��ȵģ����ױ��������Ӵ��⻬�Ҳ�©�����ֽ�ͨ��Դ������˿�Ը������建�����ȣ�
��ͼ��ʾ��һ������ֱ���ã���һ����Ϊm�Ļ����ڸ��ڷ����һ�������������壬�����ĵײ���װ��һ������˿���õ��ߺ�����Դ��������֪���ױںͻ������Ǿ��ȵģ����ױ��������Ӵ��⻬�Ҳ�©�����ֽ�ͨ��Դ������˿�Ը������建�����ȣ� ��2013?�������ģ����ͼ��ʾ��һ������ֱ���ţ���������һ�������ɺ��ԵĻ�������һ���������������������ڣ����������ױ���Ħ����ͨ���������ַ�ʽ����ʹ�������������С��������
��2013?�������ģ����ͼ��ʾ��һ������ֱ���ţ���������һ�������ɺ��ԵĻ�������һ���������������������ڣ����������ױ���Ħ����ͨ���������ַ�ʽ����ʹ�������������С��������