��Ŀ����
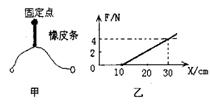
��6�֣���̽�����������ٶȱ仯�Ĺ�ϵ����ʵ��װ����ͼ��ʾ������ֹ��С����һ����Ƥ�������µ���ʱ����Ƥ���С�����Ĺ���ΪW������2����3����ȫ��ͬ����Ƥ���һ����е�2�Ρ���3��ʵ��ʱ��ʹÿ��ʵ������Ƥ���쳤�ij��ȶ�����һ�¡�ÿ��ʵ����С����õ��ٶ��ɴ���ʱ�������ֽ�������
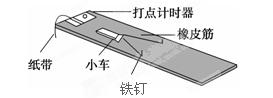
(1)��ľ��ˮƽ���ã�С����������Ƥ���������˶�����С���ٶ����ʱ��������Ƥ��������״̬��С�����ڵ�λ��(��С��Ϊ�ʵ�)������˵����ȷ��________��
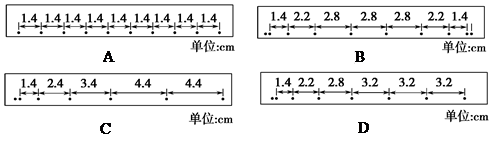
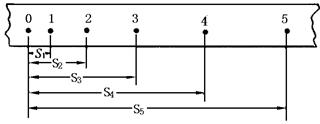
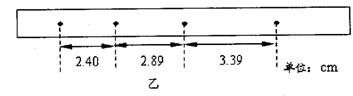
(2)����4��ֽ����Щ������̽�����������ٶȱ仯��ϵ��ʵ���У���ȷ�������ܵõ���ֽ��________��

(1)��ľ��ˮƽ���ã�С����������Ƥ���������˶�����С���ٶ����ʱ��������Ƥ��������״̬��С�����ڵ�λ��(��С��Ϊ�ʵ�)������˵����ȷ��________��
| A����Ƥ����쳤״̬ |
| B����Ƥ���ԭ��״̬ |
| C��С�����������������ߴ� |
| D��С���ѹ��������������� |

��1�� A (3��) (2) BD ��3�֣�
�����������1������ʵ����ʵ��ʱ����Ƥ����ԭ���������������Ҫ��ƽ��Ħ��������Ƥ����������ں�������Ƥ��������ϣ�������Ƥ���ָ�ԭ��ʱ��С���ٶȴﵽ���ʱС����û�е����������������ߣ���A��ȷ��BCD����ѡA��
��2������С���ȼ��٣��м����٣������٣��ʸ����˶��ص㣬�����ҵ�����������ֽ����Bֽ������Ҫ��ֽ��Ҳ���Ի�δ�ﵽ���٣���Dֽ��Ҳ���ܡ�

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
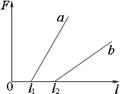
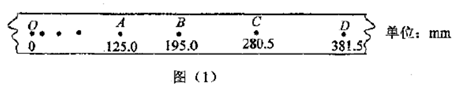
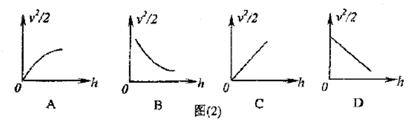

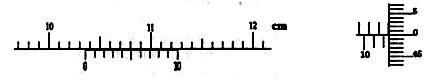

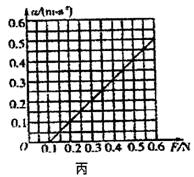
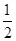 kx,����W=
kx,����W=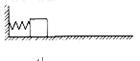

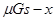 ͼ�� B��
ͼ�� B��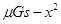 ͼ��
ͼ��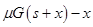 ͼ�� D��
ͼ�� D�� ͼ��
ͼ��