��Ŀ����
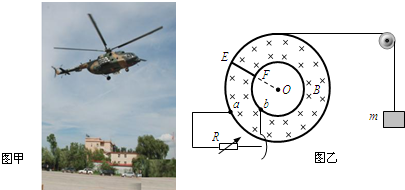
3�� ��ͼ��ʾ����һ�о����������˶���װ��ͼ������I���д�ֱֽ������ĴŸ�Ӧǿ��ΪB1����ǿ�ų������������ǿ�糡��ƽ�а���Ϊ2R�����㹻��������II����Բ������Ϊ��0��R�����뾶ΪR��Բ�δų����Ÿ�Ӧǿ��B2=$\frac{m��}{qR}$����ֱֽ�����⣬����Ϊm�������Ϊ+q���ٶȲ�ͬ�����Ӵ������������I��������I��ֻ����x�����������Ϊ�ԣ��ֲ�����Ϊ2R��������������������ӽ��������������������
��ͼ��ʾ����һ�о����������˶���װ��ͼ������I���д�ֱֽ������ĴŸ�Ӧǿ��ΪB1����ǿ�ų������������ǿ�糡��ƽ�а���Ϊ2R�����㹻��������II����Բ������Ϊ��0��R�����뾶ΪR��Բ�δų����Ÿ�Ӧǿ��B2=$\frac{m��}{qR}$����ֱֽ�����⣬����Ϊm�������Ϊ+q���ٶȲ�ͬ�����Ӵ������������I��������I��ֻ����x�����������Ϊ�ԣ��ֲ�����Ϊ2R��������������������ӽ����������������������1������I���������İ���Ƹߣ��������ĵ��Ʋ
��2�������Ӿ�������x���λ������ʹ������Ҷ����м������������II�����˶�ʱ�䣻
��3������x���·���һ�ų��������������������������������ӱ�ɷֲ�����Ϊ4R����x�Ḻ��������������������ĴŸ�Ӧǿ�ȣ�
���� ��1���ٶ�Ϊv��x�������˶����������������������ֱ���˶����糡������������ƽ�⣬��ʽ����ó�ǿ����U=Ed����������ĵ��Ʋ
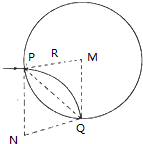
��2����������е�������Բ���˶��������������ṩ����������ţ�ٵڶ����ɿ���ù켣�뾶r=R��������ƽ����x����������ӣ�����P������������켣Բ��ΪN����PN=PM=R���������ĵ�ΪQ��PMQNΪһ���Σ�MQ��ֱ��x�ᣬ��Q������ԭ��O�غϣ�Ϊ����������������ĵ㣬����Ϊ��0��0���������ڢ������˶��켣Ϊ$\frac{1}{4}$Բ�ܣ������ڢ����˶�ʱ��Ϊ$\frac{1}{4}$���ڣ�
��3���ɣ�2��֪��ƽ�����������䵽Բ�δų������ų�����İ뾶��������Բ���˶��İ뾶�����ܽ����Ӿ۵�һ�����������ͬ�����Ӹõ��Բ�ͬ���������ͬ�������ӣ����ôų�ƫת�����ӻ�ӳ���ƽ�г��䣮������������������еĹ켣�뾶��������������ĴŸ�Ӧǿ�ȣ�
���  �⣺��1��������֪���¼�����Ƹߣ�
�⣺��1��������֪���¼�����Ƹߣ�
�� qE=qvB1��
E=vB1��
�������ĵ��Ʋ� U=E•2R=2RvB1��
��2����������е�������Բ���˶����켣�뾶Ϊr����
qvB2=m$\frac{{v}^{2}}{r}$
��������B2=$\frac{m��}{qR}$
����� r=R
������ƽ����x����������ӣ�����P������������켣Բ��ΪN����PN=PM=R���������ĵ�ΪQ��PMQNΪһ���Σ�MQ��ֱ��x�ᣬ��Q������ԭ��O�غϣ�Ϊ����������������ĵ㣬����Ϊ��0��0������֪�����ڢ������˶��켣Ϊ$\frac{1}{4}$Բ�ܣ������ڢ����˶�ʱ��Ϊ t2=$\frac{2��R}{4v}$
��3���ɣ�2��֪��ƽ�����������䵽Բ�δų������ų�����İ뾶��������Բ���˶��İ뾶�����ܽ����Ӿ۵�һ�����������ͬ�����Ӹõ��Բ�ͬ���������ͬ�������ӣ����ôų�ƫת�����ӻ�ӳ���ƽ�г��䣮
��O�㷽����������ӽ���һ��O���е�Բ�δų�����ʹ����ƽ�г��䣬�ٶ�Ϊv�������ڢ�����Բ���˶��İ뾶 r��=R��=2R
��r��=$\frac{mv}{q{B}_{3}}$���� R3=$\frac{mv}{2qR}$
��
��1������I���¼�����Ƹߣ������ĵ��Ʋ�Ϊ2RvB1��
��2�����Ӿ�������x���λ������Ϊ��0��0�����������Ҷ����м������������II�����˶�ʱ��Ϊ$\frac{2��R}{4v}$��
��3������x���·���һ�ų��������������������������������ӱ�ɷֲ�����Ϊ4R����x�Ḻ������������������ĴŸ�Ӧǿ��Ϊ$\frac{mv}{2qR}$��
���� �����������ڴų�������Բ���˶������⣮�ڴų���Բ���˶����÷����ǻ��켣���ɼ���֪ʶ��뾶��

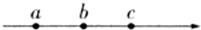 ��ͼ��ʾ��a��b��c��һ���糡���ϵ����㣬�糡�ߵķ�����a��c��a��b��������b��c����룬��ϕa��ϕb��ϕc��Ea��Eb��Ec�ֱ��ʾa��b��c����ĵ��ƺͳ�ǿ�������ж���������
��ͼ��ʾ��a��b��c��һ���糡���ϵ����㣬�糡�ߵķ�����a��c��a��b��������b��c����룬��ϕa��ϕb��ϕc��Ea��Eb��Ec�ֱ��ʾa��b��c����ĵ��ƺͳ�ǿ�������ж���������| A�� | ϕa��ϕb��ϕc | B�� | Ea��Eb��Ec | C�� | ϕa-ϕb=ϕb-ϕa | D�� | Ea=Eb=Ec |
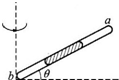 ��ͼ��ʾ��һ֧���˷�յIJ�������б���ã�������һ��ˮ���������˸������һ���������������壬�����������ʹˮ������a���ƶ����ǣ�������
��ͼ��ʾ��һ֧���˷�յIJ�������б���ã�������һ��ˮ���������˸������һ���������������壬�����������ʹˮ������a���ƶ����ǣ�������| A�� | ��˳ʱ�뷽����ת�������ܣ�ʹ�ȽDZ�С | |
| B�� | ���֦ȽDz��䣬ʹ�����ܼ������� | |
| C�� | ʹ�������¶����� | |
| D�� | �ƹ�b�˵���ֱ��ת�� |
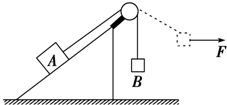 ��ͼ��ʾ��б��������ڴֲ�ˮƽ�����ϣ����Aͨ����������ֵ�����ϸ�������B���ӣ�ϵͳ���ھ�ֹ״̬���ֶ�Bʩ��һˮƽ��FʹB�������˶���ʹ����ƫ����ֱ����һ���Ƕȣ��ڴ˹��������A��б����ʼ�մ��ھ�ֹ״̬��������
��ͼ��ʾ��б��������ڴֲ�ˮƽ�����ϣ����Aͨ����������ֵ�����ϸ�������B���ӣ�ϵͳ���ھ�ֹ״̬���ֶ�Bʩ��һˮƽ��FʹB�������˶���ʹ����ƫ����ֱ����һ���Ƕȣ��ڴ˹��������A��б����ʼ�մ��ھ�ֹ״̬��������| A�� | б��������A��Ħ����һֱ���� | B�� | �����б�����֧����һֱ���� | ||
| C�� | �����б�����Ħ����һֱ���� | D�� | �����б�����֧�������ֲ��� |
| A�� | ���������Է��شӵ������崫���������� | |
| B�� | �����ۻ�ʱ��������������ƽ������һ������ | |
| C�� | ������¶����ߣ���ʾ���������з��ӵĶ��ܶ����� | |
| D�� | ����ͨ������ʹ���跢�ȣ�����Ϊ��Դ�͵����ġ��ȴ��ݡ� |
 ijͬѧҪ����һ�²����Ƴ�Բ������裬���IJ������£�
ijͬѧҪ����һ�²����Ƴ�Բ������裬���IJ������£�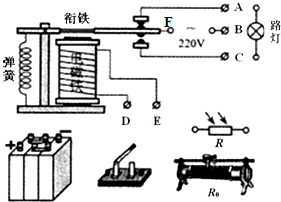 ��ͼ��ʾ�ǽֵ�·���Զ�����ģ���·�����ý���F��B֮���������ԴΪ·�ƹ��磬����ֱ����ԴΪ��������磮R�ǹ������裬�й���ʱR��С��R0�ǻ������������ڵ�·�������������ã�ͬʱ�����Ե��ڿ��Ƶ�·�������ȣ�
��ͼ��ʾ�ǽֵ�·���Զ�����ģ���·�����ý���F��B֮���������ԴΪ·�ƹ��磬����ֱ����ԴΪ��������磮R�ǹ������裬�й���ʱR��С��R0�ǻ������������ڵ�·�������������ã�ͬʱ�����Ե��ڿ��Ƶ�·�������ȣ�