��Ŀ����
��̽�������ٶ������������Ĺ�ϵ���Ļ�У�
��1����ͨ��Դ����ֽ��(������)��ʼ�˶���������������ʱ���ϵӦ����( )
A���Ƚ�ͨ��Դ�����ͷ�ֽ�� B�����ͷ�ֽ�������ͨ��Դ
C���ͷ�ֽ����ͬʱ��ͨ��Դ D���Ƚ�ͨ��Դ�����ͷ�ֽ��������
��2�����ڴ���ʱ�����ʱ�����ij��̣���λͬѧ���Է������Լ��Ŀ�����������ȷ����( )
A����Դ��ѹԽ�ߣ�ͨ���ĵ���Խǿ������ٶȾͻ�Խ�죬ÿ���������ʱ�����ͻ�Խ��
B������ֽ���ٶ�Խ��ֽ���˶�Խ�죬����ٶȾͻ�Խ�죬ÿ������������ʱ�����ͻ�Խ��
C������ʱ��ÿ���������ʱ�����ɵ�Դ��Ƶ�ʾ��������Դ��ѹ��ֽ�����ٶ���
D�����϶�����
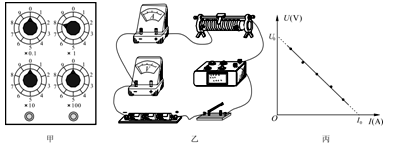
��3��ijͬѧ�ڽ�ͨ��Դ����ʵ��֮ǰ����ʵ��������װ��ͼ��ʾ������ָ����װ���еĴ������֮��������������
a.
b.
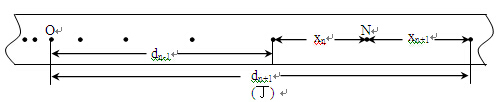
��4������ʵ��װ�ú�ͬѧ˳���������ʵ�顣��ͼ������ʵ���еõ���һ��ֽ����ÿ�����ʱ��ȡһ�������㡣���������ʱ����Ϊ s����ͼ�е����ݿ����С���ļ��ٶ�aΪ m/s2.
��5����ͬѧ��ʵ���б����������䣬�õ���С�����ٶ��������仯��һ�����ݣ����±���ʾ
| ʵ����� | ���ٶ� a/ m��s��2 | С�������������� m/kg | С���������������ĵ��� m��1 /kg��1 |
| 1 | 0.29 | 0.20 | 5.0 |
| 2 | 0.25 | 0.25 | 4.0 |
| 3 | 0.22 | 0.30 | 3.3 |
| 4 | 0.18 | 0.35 | 2.9 |
| 5 | 0.16 | 0.40 | 2.5 |

��1��A��2��C ��3������ʱ����Ӧʹ�øɵ�أ�Ӧʹ�ý�����Դ��ʵ����û��ƽ��С����Ħ������С����ʼλ�������ʱ��̫Զ ��ֻҪ������е����㼴�ɡ���
��4��0.1, 0.20 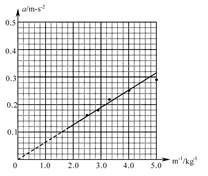
���ֱ��� ���㡱��������ȷ���������ֲ���ͼ�߸�����ͼ�߹⻬ȷ�÷֡�����a��mͼ��IJ��÷֡�
���������������1����ʼ��¼ʱ��Ӧ�ȸ�����ʱ��ͨ���㣬Ȼ���ͷ�ֽ����ֽ���������壩��ʼ�˶�������ȷſ�ֽ����ʼ�˶����ٽ�ͨ����ʱʱ���ĵ�Դ�����������˶��Ͽ죬���������ݵIJɼ��ʹ��������ʵ������ϴ����ͬʱ�ȴ�����ͷ�ֽ��������ʹ����ȶ������ֽ�������ʣ�����ʹֽ���ϴ����㣬��A��ȷ��
��2������ʱ����ʹ�ý�����Դ���������������뽻�����������ͬ�����ѹ���ٶȵ��أ���C��ȷ��
��3����ʵ���е�Ŵ���ʱ��������4��6V�Ľ�����Դ�����ʱ��������220V������Դ������ֱ����Դ��Ϊ�˾����ܵ�����ֽ�����ͷ�ǰС��Ӧ��������ʱ�����ڱ�ʵ����������Ϊ���ӵ����������������ܵĺ�����������ʵ��ǰҪ����ƽ��Ħ������
��4��������������֮���ʱ����Ϊ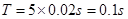 �����һ�������㵽�ڶ���������֮��ľ���Ϊ
�����һ�������㵽�ڶ���������֮��ľ���Ϊ ���Ժ���ηֱ�Ϊ
���Ժ���ηֱ�Ϊ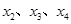 �������ȱ���ֱ���˶������۹�ʽ
�������ȱ���ֱ���˶������۹�ʽ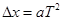 ����������ٶȵĴ�С���ã�
����������ٶȵĴ�С���ã�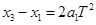 ��
��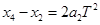 ��Ϊ�˸���ȷ�������ٶȣ����Ƕ��������ٶ�ȡƽ��ֵ�ã�
��Ϊ�˸���ȷ�������ٶȣ����Ƕ��������ٶ�ȡƽ��ֵ�ã�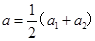 ��
��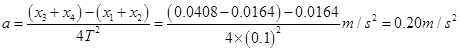
��5��Ϊ����ֱ������ı�������ٶȺ�������ϵ��������Ҫ����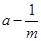 ͼ����ͼ��ʾ��
ͼ����ͼ��ʾ��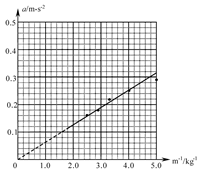
���㣺̽�������ٶ������������Ĺ�ϵ��

̽������ԭ����ֹ���������Ĺ��������õ��ٶȵĹ�ϵ��ʵ��װ����ͼ��ʾ��ʵ����Ҫ�������£� 
��1���跨����Ƥ���С�����Ĺ��ֱ�ΪW��2W��3W����
��2����������ʱ�������ֽ�������С�����ٶ�v1��v2��v3������
��3������W��v��ͼ��
��4������W��vͼ�����W��vͼ����һ��ֱ�ߣ�����W��v���������ֱ�ߣ��ɿ����Ƿ����W��v2��W��v3��W�� �ȹ�ϵ��
�ȹ�ϵ��
���¹��ڸ�ʵ���˵������һ���ȷ������________��
| A����ʵ���跨����Ƥ���С�����Ĺ��ֱ�ΪW��2W��3W���������õķ�����ѡ��ͬ������Ƥ�����ÿ��ʵ����ʹ��Ƥ������ij��ȱ���һ�£�����1����Ƥ�����ʵ��ʱ����Ƥ���С�����Ĺ�ΪW����2����3��������Ƥ���һ����е�2�Ρ���3�Ρ���ʵ��ʱ����Ƥ���С�����Ĺ��ֱ���2W��3W���� |
| B��С���˶��л��ܵ������������ķ���������ʹľ���ʵ���б |
| C��ijͬѧ��һ��ʵ���У��õ�һ����¼ֽ����ֽ���ϴ���ĵ㣬�����ܡ��м��裮�������������ԭ������û��ʹľ����б�����̫С |
| D�����ݼ�¼ֽ���ϴ���ĵ㣬��С����õ��ٶȵķ���������ֽ���ϵ�һ�㵽���һ��ľ��������м��� |
ijͬѧ������ֱ����С���Ƶ����Ƭ��֤��е���غ㶨�ɡ�Ƶ����ÿ��0.05s ����һ�Σ��ú��̶ȳ߲����������ʱ��С�������ĸ߶ȷֱ�Ϊs1=26.3cm��s2=23.68cm��s3=21.16cm��s4=18.66cm��s5=16.04cm����ͬѧͨ������õ���ͬʱ�̵��ٶ����±���ʾ�������������ٶ�g=9.80m/s2��С������m=0.10kg����
| ʱ�� |  |  |  |  |
| �ٶȣ�m/s�� | | 4.48 | 3.98 | 3.47 |

��1����������߶ȵ���������в�������Ч���ֶ���Ҫ����� �Σ�Ӧ���� cm��
��2����Ƶ����Ƭ�ϵ����ݼ���t2ʱ��С����ٶ�
 ��__________m/s��(������������λ��Ч���֣�
��__________m/s��(������������λ��Ч���֣���3����t2��t5ʱ���ڣ�������������
 ��_________J�����ܼ�����
��_________J�����ܼ����� ��________J����������������λ��Ч���֣�
��________J����������������λ��Ч���֣���4������������ķ�Χ�ڣ���
 ��
�� ������ȣ��Ӷ���֤�˻�е���غ㶨�ɡ���������������
������ȣ��Ӷ���֤�˻�е���غ㶨�ɡ��������������� _____
_____ ��ѡ�>������<����������������ֽ������Ҫԭ���� ��
��ѡ�>������<����������������ֽ������Ҫԭ���� �� 






 ��
�� ��
��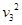 ��������
��������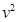 ���������ʾ�������������Ĺ�W������
���������ʾ�������������Ĺ�W������ ͼ����ͼ���������
ͼ����ͼ��������� ������㣬������ͼ�ߣ�
������㣬������ͼ�ߣ� ��40.00cm�����������������Ĺ�
��40.00cm�����������������Ĺ� ��________J����gȡ
��________J����gȡ �����������λ��Ч���֣�
�����������λ��Ч���֣�

 = �� �������ַ����= J����С�������2λ��Ч���֣�
= �� �������ַ����= J����С�������2λ��Ч���֣�