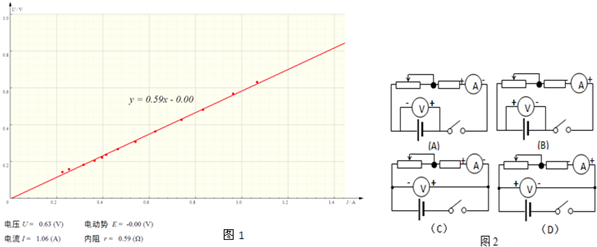
��Ŀ����
11�� ��ͼ��һ����ϸ���ȡ��ڱڹ⻬����ֱ���õIJ������¶��ܷ⣬�϶�����һ�����ף������²���������סһ���������壨����Ϊ�������壩�������¶�ΪT1��ʼʱt�������Ϸ������建��������������Ϸ���ѹǿ�ﵽpoʱ�������·���������ΪV1�������Ϸ������ܵ��ݻ�Ϊ26V1������������ѹǿΪ0.5po�����������Ϸ������ղ��ܷ��������������й�����ʼ�ձ��ֲ���Ȼ���ܷ�����建��������
��ͼ��һ����ϸ���ȡ��ڱڹ⻬����ֱ���õIJ������¶��ܷ⣬�϶�����һ�����ף������²���������סһ���������壨����Ϊ�������壩�������¶�ΪT1��ʼʱt�������Ϸ������建��������������Ϸ���ѹǿ�ﵽpoʱ�������·���������ΪV1�������Ϸ������ܵ��ݻ�Ϊ26V1������������ѹǿΪ0.5po�����������Ϸ������ղ��ܷ��������������й�����ʼ�ձ��ֲ���Ȼ���ܷ�����建����������1�����������������ܶ���ʱ������¶ȣ�
��2���������¶ȴﵽ1.8T1ʱ�����ѹǿ��
���� ��1���������Ϸ������建������������������±仯���жϻ����Ƿﲣ���ܶ��ˣ���ȷ������仯���̣�
��2���ɵ�1���н��ȷ��2�еı仯���̣�
��� �⣺��1�����Ϸ����������ʱ���������ΪV2��������ñ仯ǰ��P1=P0+0.5P0=1.5P0�����Ϊ��V1���仯��P2=0.5P0�������V2���в��������P1V1=P2V2�ã�V2=3V1��3.6V1���ʻ��������������ܶ���ʱ�����¶�Ҳ�����仯������������״̬���̣�
$\frac{{P}_{1}{V}_{1}}{{T}_{1}}$=$\frac{{P}_{2}{V}_{2}}{{T}_{2}}$��
�ã�
T2=1.2T1��
��2���ɣ�1����֪1.8T1ʱ�����ѵ��ﶥ�ˣ����ʱ���¶�ΪT3�����Ϊ3.6V1�����������巽�̣�
$\frac{{P}_{1}{V}_{1}}{{T}_{1}}=\frac{{P}_{3}{V}_{3}}{{T}_{3}}$��
�ã�
P3=0.75P0
�𣺣�1�����������������ܶ���ʱ������¶���1.2T��
��2���������¶ȴﵽ1.8T1ʱ�����ѹǿ��0.75p0��
���� ��������״̬�仯���⣬�ؼ����������״̬������ȷ���Ǻ��ֱ仯���̣����з�����⣮

��ϰ��ϵ�д�
�����Ŀ
2��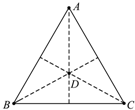 ����ռ���һЩ��ϵ������������ľ���dz�ңԶ�����Ժ���������ϵ�����ǵ����ã������Ŀ��������һ�ȶ���ϵ���������������������У�������������A��B��Cλ�ڱ߳�Ϊa���������ε����������ϣ������Բ���������Բ���˶������Ŀ�����Dλ�����������ԲԲ�ģ��Ŀ������������Ϊm��������������ΪG��������˵����ȷ���ǣ�������
����ռ���һЩ��ϵ������������ľ���dz�ңԶ�����Ժ���������ϵ�����ǵ����ã������Ŀ��������һ�ȶ���ϵ���������������������У�������������A��B��Cλ�ڱ߳�Ϊa���������ε����������ϣ������Բ���������Բ���˶������Ŀ�����Dλ�����������ԲԲ�ģ��Ŀ������������Ϊm��������������ΪG��������˵����ȷ���ǣ�������
 ����ռ���һЩ��ϵ������������ľ���dz�ңԶ�����Ժ���������ϵ�����ǵ����ã������Ŀ��������һ�ȶ���ϵ���������������������У�������������A��B��Cλ�ڱ߳�Ϊa���������ε����������ϣ������Բ���������Բ���˶������Ŀ�����Dλ�����������ԲԲ�ģ��Ŀ������������Ϊm��������������ΪG��������˵����ȷ���ǣ�������
����ռ���һЩ��ϵ������������ľ���dz�ңԶ�����Ժ���������ϵ�����ǵ����ã������Ŀ��������һ�ȶ���ϵ���������������������У�������������A��B��Cλ�ڱ߳�Ϊa���������ε����������ϣ������Բ���������Բ���˶������Ŀ�����Dλ�����������ԲԲ�ģ��Ŀ������������Ϊm��������������ΪG��������˵����ȷ���ǣ�������| A�� | ����A�ܵ���������Ϊ��3+$\sqrt{3}$��$\frac{{G{m^2}}}{a^2}$ | B�� | ����A�ܵ���������Ϊ��3+2$\sqrt{3}$��$\frac{{G{m^2}}}{a^2}$ | ||
| C�� | ����B���е�����Ϊ2��a$\sqrt{\frac{a}{{��1+3\sqrt{3}��Gm}}}$ | D�� | ����B���е�����Ϊ2��a$\sqrt{\frac{a}{{��3+\sqrt{3}��Gm}}}$ |
19��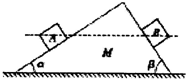 ��ͼ��ʾ��һ������Ϊ90���б����M����ˮƽ���ϣ����ĵ���ֲڣ���б��⻬������б����ˮƽ��ļнǷֱ�Ϊ�����£��Ҧ����£���������ȵ�A��B����С����ͬʱ��б����ͬһ�߶ȴ���ֹ�ͷţ��������黬��б��˵Ĺ����У�Mʼ�ձ��־�ֹ��������
��ͼ��ʾ��һ������Ϊ90���б����M����ˮƽ���ϣ����ĵ���ֲڣ���б��⻬������б����ˮƽ��ļнǷֱ�Ϊ�����£��Ҧ����£���������ȵ�A��B����С����ͬʱ��б����ͬһ�߶ȴ���ֹ�ͷţ��������黬��б��˵Ĺ����У�Mʼ�ձ��־�ֹ��������
 ��ͼ��ʾ��һ������Ϊ90���б����M����ˮƽ���ϣ����ĵ���ֲڣ���б��⻬������б����ˮƽ��ļнǷֱ�Ϊ�����£��Ҧ����£���������ȵ�A��B����С����ͬʱ��б����ͬһ�߶ȴ���ֹ�ͷţ��������黬��б��˵Ĺ����У�Mʼ�ձ��־�ֹ��������
��ͼ��ʾ��һ������Ϊ90���б����M����ˮƽ���ϣ����ĵ���ֲڣ���б��⻬������б����ˮƽ��ļнǷֱ�Ϊ�����£��Ҧ����£���������ȵ�A��B����С����ͬʱ��б����ͬһ�߶ȴ���ֹ�ͷţ��������黬��б��˵Ĺ����У�Mʼ�ձ��־�ֹ��������| A�� | �����б�����֧����С����������������� | |
| B�� | �����黬��б������õ�ʱ����ͬ | |
| C�� | �����黬��б���ʱ������˲ʱ������ͬ | |
| D�� | �����б�����֧����������������������� |
6��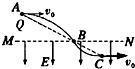 ��ͼ��ʾ���ڿռ���ˮƽ��MN���·�������ֱ���µ���ǿ�糡������Ϊm�Ĵ���С��Q��ˮƽ��MN���Ϸ�A����һ�����ٶ�v0ˮƽ�׳�����B�������ǿ�糡������C��ʱ�ٶȷ���ǡ��ˮƽ������A��B��C������ͬһֱ���ϣ����߶�AB=2BC���ɴ˿�֪��������
��ͼ��ʾ���ڿռ���ˮƽ��MN���·�������ֱ���µ���ǿ�糡������Ϊm�Ĵ���С��Q��ˮƽ��MN���Ϸ�A����һ�����ٶ�v0ˮƽ�׳�����B�������ǿ�糡������C��ʱ�ٶȷ���ǡ��ˮƽ������A��B��C������ͬһֱ���ϣ����߶�AB=2BC���ɴ˿�֪��������
 ��ͼ��ʾ���ڿռ���ˮƽ��MN���·�������ֱ���µ���ǿ�糡������Ϊm�Ĵ���С��Q��ˮƽ��MN���Ϸ�A����һ�����ٶ�v0ˮƽ�׳�����B�������ǿ�糡������C��ʱ�ٶȷ���ǡ��ˮƽ������A��B��C������ͬһֱ���ϣ����߶�AB=2BC���ɴ˿�֪��������
��ͼ��ʾ���ڿռ���ˮƽ��MN���·�������ֱ���µ���ǿ�糡������Ϊm�Ĵ���С��Q��ˮƽ��MN���Ϸ�A����һ�����ٶ�v0ˮƽ�׳�����B�������ǿ�糡������C��ʱ�ٶȷ���ǡ��ˮƽ������A��B��C������ͬһֱ���ϣ����߶�AB=2BC���ɴ˿�֪��������| A�� | С������� | |
| B�� | �糡����СΪ3mg | |
| C�� | С���A��B�˶���ʱ����ڴ�B��C���˶�ʱ�� | |
| D�� | С���A��B���B��C���ٶȱ仯����� |
3��2014��10��22�գ�ִ��̽�¹������ڷ�������������϶���ŷ������������������Ƿ������ķ��䣮��Ϥ�������϶�ϵ�з������״���Ҫ���ص����һ������Ϊ�϶���ŷ����ṩ�������ݣ������ҹ�������ҵ����һ�ɹ������϶���ŷ�����������Χ��������˶����Կ�������Բ���˶�����������������֪����������̽�����Ҫ�������������������Ҫ֪�����������У�������
| A�� | ��������������������������İ뾶 | |
| B�� | ����İ뾶�͡��������������������˶������� | |
| C�� | ���������������������˶������ں���뾶 | |
| D�� | ��������������������������İ뾶�͡��������������������˶������� |
20�����и���˵������ȷ���ǣ�������
| A�� | Һ���������������ԭ���ǣ�Һ��������ӽ��ܼ������Ӽ��������ڳ��� | |
| B�� | PM2.5 ��������ֱ�����ڻ�С��2.5����������� �ڿ����е��˶����ڷ������˶� | |
| C�� | �ڸ��־����У�ԭ�� ������ӡ����ӣ� ���ǰ���һ���Ĺ������еģ����пռ��ϵ������� | |
| D�� | һ��֮������������¶����ߣ��������ѹǿ���䣬����������ڵ�λʱ��ײ�������ڵ�λ����Ĵ���һ������ | |
| E�� | ��ʹ�������Ӽ�ľ����ɺ�Զ ��r��10-9m�� ��С�������ٿ����Ĺ����У����Ӽ����������������С�������� |
1��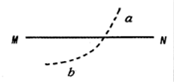 ��ͼ��ʾ��MN����һ��������Q�����ĵ糡�е�һ���糡�ߣ�һ�������������+q����糡���ڵ糡������������һ�������˶����Ⱥ�ͨ��a��b���㣬�������ӵ�������������
��ͼ��ʾ��MN����һ��������Q�����ĵ糡�е�һ���糡�ߣ�һ�������������+q����糡���ڵ糡������������һ�������˶����Ⱥ�ͨ��a��b���㣬�������ӵ�������������
 ��ͼ��ʾ��MN����һ��������Q�����ĵ糡�е�һ���糡�ߣ�һ�������������+q����糡���ڵ糡������������һ�������˶����Ⱥ�ͨ��a��b���㣬�������ӵ�������������
��ͼ��ʾ��MN����һ��������Q�����ĵ糡�е�һ���糡�ߣ�һ�������������+q����糡���ڵ糡������������һ�������˶����Ⱥ�ͨ��a��b���㣬�������ӵ�������������| A�� | ������a��ļ��ٶȴ�����b��ļ��ٶ� | |
| B�� | ��MN����λ�����ϵĵ��Ʊ仯����С | |
| C�� | ������a��Ķ���Eka������b��Ķ���Ekb | |
| D�� | ������a��ĵ�����EPaС����b��ĵ�����Epb |