��Ŀ����
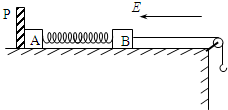 ��ͼ��ʾ������P�̶����㹻�ߵ�ˮƽ�����ϣ�С���A��B��С�ɺ��ԣ����Ƿֱ����+QA��+QB�ĵ�����������ֱ�ΪmA��mB��������ɾ�Ե���ᵯ��������һ�������쳤������������֣�һ����B���ӣ���һ����������С��������װ�ô��ڳ�ǿΪE������ˮƽ�������ǿ�糡�У�A��B��ʼʱ��ֹ����֪���ɵľ���ϵ��Ϊk������һ��Ħ����A��B��Ŀ�������A��B������������ֲ��䣬Bһֱ��ˮƽ�����˶��Ҳ����������֣�����
��ͼ��ʾ������P�̶����㹻�ߵ�ˮƽ�����ϣ�С���A��B��С�ɺ��ԣ����Ƿֱ����+QA��+QB�ĵ�����������ֱ�ΪmA��mB��������ɾ�Ե���ᵯ��������һ�������쳤������������֣�һ����B���ӣ���һ����������С��������װ�ô��ڳ�ǿΪE������ˮƽ�������ǿ�糡�У�A��B��ʼʱ��ֹ����֪���ɵľ���ϵ��Ϊk������һ��Ħ����A��B��Ŀ�������A��B������������ֲ��䣬Bһֱ��ˮƽ�����˶��Ҳ����������֣�������1����ʼA��B��ֹʱ������P�����A����������С��
��2������С���Ϲ�����ΪM�����C���ɾ�ֹ�ͷţ������C���䵽������ʱ���A�Ե���P��ѹ��ǡ��Ϊ�㣬�����C����������룻
��3����C��������Ϊ2M����A���뿪����Pʱ��B���ٶȶ��
��������1����ʼA��B��ֹʱ����AB����һ�����壬����ƽ��������������P�����A����������С��
��2����ʼ״̬���ɴ���ѹ��״̬���α���Ϊ x1�����A�Ե���P��ѹ��ǡΪ�㣬�������뿪P����ʱA��B��C��ͬ������ɵ�ϵͳ��ͬ˲�侲ֹ��A���ܵ糡���뵯�ɵĵ�����С��ȣ������෴�����ɵ��쳤��x2������֮��Ҳ����C������½����룬�˹�����C�������ܵļ�����ǡ���ڵ��ɵ���������B�����ܵ�����֮�ͣ�
��3����C��������Ϊ2M����A���뿪����Pʱ�����ɵ��쳤����Ϊx2������ʱA���徲ֹ��B��C��������ٶ�����Ҳ�Ϊ�㣬�˹�����C�����������ܼ���������B�����е�ܺ͵����ܵ����������ɵ��ɵ�������������ϵͳ���ܵ�����֮�ͣ�
��2����ʼ״̬���ɴ���ѹ��״̬���α���Ϊ x1�����A�Ե���P��ѹ��ǡΪ�㣬�������뿪P����ʱA��B��C��ͬ������ɵ�ϵͳ��ͬ˲�侲ֹ��A���ܵ糡���뵯�ɵĵ�����С��ȣ������෴�����ɵ��쳤��x2������֮��Ҳ����C������½����룬�˹�����C�������ܵļ�����ǡ���ڵ��ɵ���������B�����ܵ�����֮�ͣ�
��3����C��������Ϊ2M����A���뿪����Pʱ�����ɵ��쳤����Ϊx2������ʱA���徲ֹ��B��C��������ٶ�����Ҳ�Ϊ�㣬�˹�����C�����������ܼ���������B�����е�ܺ͵����ܵ����������ɵ��ɵ�������������ϵͳ���ܵ�����֮�ͣ�
����⣺��1����ʼA��B��ֹʱ��AB����ƽ�⣬ˮƽ�����У�
N=E��QA+QB��
��2����ʼʱ�����α���Ϊx1��
��ƽ��������kx1=EQB �ã�x1=
����
�赱A���뿪����ʱ���ɵ��α���Ϊx2��
�ɣ�kx2=EQA �ã�x2=
����
��C�½���������Ϊ��h=x1+x2����
�ɢ١���ʽ�ɽ��h=
��QA+QB������
��3���������غ㶨�ɿ�֪��C����h�����У�C�������ܵļ���������B�ĵ����ܵ������͵��ɵ������ܵ������Լ�ϵͳ���ܵ�����֮��
��C������ΪMʱ��Mgh=QBE?h+��E������
��C������Ϊ2Mʱ����A���뿪����ʱB���ٶ�ΪV������
2Mgh=QBEh+��E��+
��2M+mB��V2����
�ɢܡ���ʽ�ɽ��A���뿪PʱB���ٶ�Ϊ��V=
�𣺣�1����ʼA��B��ֹʱ������P�����A����������СΪE��QA+QB����
��2�����C�����������Ϊ
��QA+QB����
��3����C��������Ϊ2M����A���뿪����Pʱ��B���ٶ�Ϊ
��
N=E��QA+QB��
��2����ʼʱ�����α���Ϊx1��
��ƽ��������kx1=EQB �ã�x1=
| EQB |
| k |
�赱A���뿪����ʱ���ɵ��α���Ϊx2��
�ɣ�kx2=EQA �ã�x2=
| EQA |
| k |
��C�½���������Ϊ��h=x1+x2����
�ɢ١���ʽ�ɽ��h=
| E |
| k |
��3���������غ㶨�ɿ�֪��C����h�����У�C�������ܵļ���������B�ĵ����ܵ������͵��ɵ������ܵ������Լ�ϵͳ���ܵ�����֮��
��C������ΪMʱ��Mgh=QBE?h+��E������
��C������Ϊ2Mʱ����A���뿪����ʱB���ٶ�ΪV������
2Mgh=QBEh+��E��+
| 1 |
| 2 |
�ɢܡ���ʽ�ɽ��A���뿪PʱB���ٶ�Ϊ��V=
|
�𣺣�1����ʼA��B��ֹʱ������P�����A����������СΪE��QA+QB����
��2�����C�����������Ϊ
| E |
| k |
��3����C��������Ϊ2M����A���뿪����Pʱ��B���ٶ�Ϊ
|
������������̽Ϸ��ӣ��漰���ܹ�ϵ�࣬�е������ܡ������ܡ��������ܵ�֮���ת����ȫ�濼����ѧ���ۺϷ������������ͶԹ��ܹ�ϵ�����⼰Ӧ�ã��ѶȽϴ���������Ŀ�ڷ��������У�Ҫ����Ϊ���Ѹ��ӹ��̣��ֽ�Ϊ���С���̷�����ͬʱҪ��ȷ�������������Ū��ϵͳ�˶�״̬�Լ����ܹ�ϵ��

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
�����Ŀ
 ��ͼ��ʾ������P�̶����㹻�ߵ�ˮƽ�����ϣ�С���A��B�Ĵ�С�ɺ��ԣ����Ƿֱ����+QA��+QB�ĵ�����������ֱ�ΪmA��mB��������ɾ�Ե���ᵯ��������һ�����쳤������������֣�һ����B���ӣ���һ����������С��������װ�ô��ڵ糡ǿ��ΪE������ˮƽ�������ǿ�糡�У�A��B��ʼʱ��ֹ����֪���ɵľ���ϵ��Ϊk������һ��Ħ����A��B��Ŀ�����������A��B������������ֲ��䣬B�����������֣�
��ͼ��ʾ������P�̶����㹻�ߵ�ˮƽ�����ϣ�С���A��B�Ĵ�С�ɺ��ԣ����Ƿֱ����+QA��+QB�ĵ�����������ֱ�ΪmA��mB��������ɾ�Ե���ᵯ��������һ�����쳤������������֣�һ����B���ӣ���һ����������С��������װ�ô��ڵ糡ǿ��ΪE������ˮƽ�������ǿ�糡�У�A��B��ʼʱ��ֹ����֪���ɵľ���ϵ��Ϊk������һ��Ħ����A��B��Ŀ�����������A��B������������ֲ��䣬B�����������֣�